Obscure functions: the location-function relationship of obscurins
- PMID: 28510116
- PMCID: PMC5498325
- DOI: 10.1007/s12551-017-0254-x
Obscure functions: the location-function relationship of obscurins
Abstract
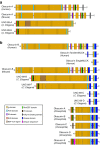
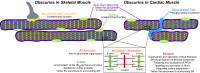
The obscurin family of polypeptides is essential for normal striated muscle function and contributes to the pathogenesis of fatal diseases, including cardiomyopathies and cancers. The single mammalian obscurin gene, OBSCN, gives rise to giant (∼800 kDa) and smaller (∼40-500 kDa) proteins that are composed of tandem adhesion and signaling motifs. Mammalian obscurin proteins are expressed in a variety of cell types, including striated muscles, and localize to distinct subcellular compartments where they contribute to diverse cellular processes. Obscurin homologs in Caenorhabditis elegans and Drosophila possess a similar domain architecture and are also expressed in striated muscles. The long sought after question, "what does obscurin do?" is complex and cannot be addressed without taking into consideration the subcellular distribution of these proteins and local isoform concentration. Herein, we present an overview of the functions of obscurins and begin to define the intricate relationship between their subcellular distributions and functions in striated muscles.
Keywords: Cardiac muscle; Cardiomyopathy; Molecular scaffold; Skeletal muscle; UNC-89.
Conflict of interest statement
Conflict of interest
Maegen A. Ackermann declares that none of the authors have any conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by the author.
Figures


References
-
- Agarkova I, Perriard JC (2005) The M-band: an elastic web that crosslinks thick filaments in the center of the sarcomere. Trends Cell Biol 15:477–485. doi:10.1016/j.tcb.2005.07.001 - PubMed
-
- Arimura T, Matsumoto Y, Okazaki O et al (2007) Structural analysis of obscurin gene in hypertrophic cardiomyopathy. Biochem Biophys Res Commun 362:281–287. doi:10.1016/j.bbrc.2007.07.183 - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

