A Dual Palladium and Copper Hydride Catalyzed Approach for Alkyl-Aryl Cross-Coupling of Aryl Halides and Olefins
- PMID: 28510287
- PMCID: PMC5572809
- DOI: 10.1002/anie.201703400
A Dual Palladium and Copper Hydride Catalyzed Approach for Alkyl-Aryl Cross-Coupling of Aryl Halides and Olefins
Abstract
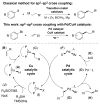
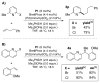
We report an efficient means of sp2 -sp3 cross coupling for a variety of terminal monosubstituted olefins with aryl electrophiles using Pd and CuH catalysis. In addition to its applicability to a range of aryl bromide substrates, this process was also suitable for electron-deficient aryl chlorides, furnishing higher yields than the corresponding aryl bromides in these cases. The optimized protocol does not require the use of a glovebox and employs air-stable Cu and Pd complexes as precatalysts. A reaction on 10 mmol scale further highlighted the practical utility of this protocol. Employing a similar protocol, a series of cyclic alkenes were also examined. Cyclopentene was shown to undergo efficient coupling under these conditions. Lastly, deuterium-labeling studies indicate that deuterium scrambling does not take place in this sp2 -sp3 cross coupling, implying that β-hydride elimination is not a significant process in this transformation.
Keywords: alkenes; copper; cross-coupling; homogeneous catalysis; palladium.
© 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



References
-
- de Meijere A, Diederich F, editors. Metal-Catalyzed Cross-Coupling Reactions. Wiley-VCH; Weinheim: 2004.
- Negishi E, editor. Handbook of Organopalladium Chemistry for Organic Synthesis. John Wiley & Sons, Inc; New York: 2002. pp. 215–994.
- Jana R, Pathak TP, Sigman MS. Chem Rev. 2011;111:1417. - PMC - PubMed
- Beller M, Bolm C, editors. Transition Metals for Organic Synthesis. Wiley-VCH; Weinheim: 2004.
-
- Miyaura N, Ishiyama T, Ishikawa M, Suzuki A. Tetrahedron Lett. 1986;27:6369.
- Miyaura N, Ishiyama T, Sasaki H, Ishikawa M, Satoh M, Suzuki A. J Am Chem Soc. 1989;111:314.
- Miyaura N, Suzuki A. Chem Rev. 1995;95:2457.
- Doucet H. Eur J Org Chem. 2008:2013.
- Lennox AJJ, Lloyd-Jones GC. Chem Soc Rev. 2014;43:412. - PubMed
-
- Brown HC, editor. Organic Syntheses via Boranes. John Wiley & Sons, Inc; New York: 2001.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

