Second-line pazopanib in patients with relapsed and refractory small-cell lung cancer: a multicentre phase II study of the Hellenic Oncology Research Group
- PMID: 28510571
- PMCID: PMC5520202
- DOI: 10.1038/bjc.2017.137
Second-line pazopanib in patients with relapsed and refractory small-cell lung cancer: a multicentre phase II study of the Hellenic Oncology Research Group
Abstract
Background: Pazopanib is a tyrosine kinase inhibitor with antiangiogenic activity. Vascular endothelial growth factor expression is increased in SCLC and is correlated with poor prognosis. The efficacy and tolerance of second-line pazopanib in SCLC was evaluated.
Patients and methods: Patients with platinum-sensitive (cohort A; n=39) and -resistant/refractory (cohort B; n=19) SCLC were enrolled in a multicentre phase II study. The primary end point was the progression-free survival rate (PFS-R) at week 8 in each cohort. Pazopanib (800 mg per day per os) was administered until progressive disease (PD). Circulating tumour cells (CTCs) were enumerated using the Cellsearch assay.
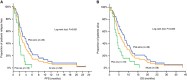
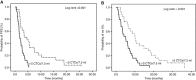
Results: All patients were evaluable for response and toxicity. In the intention-to-treat analysis, eight (13.8%) patients achieved partial response (PR) (95% confidence interval (CI): 5.0-22.7), 20 (34.5%) stable disease (SD) and 30 (51.7%) PD. Accrual in cohort B was halted because the hard-stop rule was met; in cohort A, the PFS-R was 59% (95% CI: 43.5-74.4; PR=7, SD=16). Nine (23.1%) patients received pazopanib for >6 months and 3 of them for >12 months. One pazopanib cycle resulted to a significant decrease to the number of patients with ⩾5 CTCs/7.5 ml of blood (20%) compared with baseline (50%). The median PFS and OS for all patients was 2.5 months (95% CI: 1.9-3.1 months) and 6.0 months (95% CI: 3.8-8.2 months), respectively (cohort A: PFS=3.7 months and OS=8.0 months). No unexpected toxicity was observed.
Conclusions: Second-line treatment with pazopanib in platinum-sensitive SCLC is well tolerated and resulted in promising objective responses and disease control; CTC enumeration might serve as a reliable surrogate biomarker of response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW (2004) Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 10(20): 6897–6904. - PubMed
-
- Ardizzoni A (2004) Topotecan in the treatment of recurrent small cell lung cancer: an update. Oncologist 9(Suppl 6): 4–13. - PubMed
-
- Arnold AM, Seymour L, Smylie M, Ding K, Ung Y, Findlay B, Lee CW, Djurfeldt M, Whitehead M, Ellis P, Goss G, Chan A, Meharchand J, Alam Y, Gregg R, Butts C, Langmuir P, Shepherd F (2007) Phase II study of vandetanib or placebo in small-cell lung cancer patients after complete or partial response to induction chemotherapy with or without radiation therapy: National Cancer Institute of Canada Clinical Trials Group Study BR.20. J Clin Oncol 25(27): 4278–4284. - PubMed
-
- Ciuleanu T, Samarzjia M, Demidchik Y, Beliakouski V, Rancic M, Bentsion L, Orlov V, Schaeffler A, De Jager L, Breitz B (2010) Randomized phase III study (SPEAR) of picoplatin plus best supportive care (BSC) or BSC alone in patients (pts) with SCLC refractory or progressive within 6 months after first-line platinum-based chemotherapy. Presented at the 2010 ASCO Annual Meeting 28: Suppl 15 (abstract 7002).
-
- Dowell JE, Amirkhan RH, Lai WS, Frawley WH, Minna JD (2004) Survival in small cell lung cancer is independent of tumor expression of VEGF and COX-2. Anticancer Res 24(4): 2367–2373. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

