Dose of antivenom for the treatment of snakebite with neurotoxic envenoming: Evidence from a randomised controlled trial in Nepal
- PMID: 28510574
- PMCID: PMC5446183
- DOI: 10.1371/journal.pntd.0005612
Dose of antivenom for the treatment of snakebite with neurotoxic envenoming: Evidence from a randomised controlled trial in Nepal
Abstract
Background: Currently, there is inadequate evidence on which to base clinical management of neurotoxic snakebite envenoming, especially in the choice of initial antivenom dosage. This randomised controlled trial compared the effectiveness and safety of high versus low initial antivenom dosage in victims of neurotoxic envenoming.
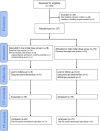
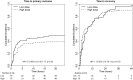
Methodology/ principal findings: This was a balanced, randomised, double-blind trial that was conducted in three health care centers located in the Terai plains of Nepal. Participants received either low (two vials) or high (10 vials) initial dosage of Indian polyvalent antivenom. The primary composite outcome consisted of death, the need for assisted ventilation and worsening/recurrence of neurotoxicity. Hourly evaluations followed antivenom treatment. Between April 2011 and October 2012, 157 snakebite victims were enrolled, of which 154 were analysed (76 in the low and 78 in the high initial dose group). Sixty-seven (43·5%) participants met the primary outcome definition. The proportions were similar in the low (37 or 48.7%) vs. high (30 or 38.5%) initial dose group (difference = 10·2%, 95%CI [-6·7 to 27·1], p = 0·264). The mean number of vials used was similar between treatment groups. Overall, patients bitten by kraits did worse than those bitten by cobras. The occurrence of treatment-related adverse events did not differ among treatment groups. A total of 19 serious adverse events occurred, including seven attributed to antivenom.
Conclusions: This first robust trial investigating antivenom dosage for neurotoxic snakebite envenoming shows that the antivenom currently used in Nepal performs poorly. Although the high initial dose regimen is not more effective than the low initial dose, it offers the practical advantage of being a single dose, while not incurring higher consumption or enhanced risk of adverse reaction. The development of new and more effective antivenoms that better target the species responsible for bites in the region will help improve future patients' outcomes.
Trial registration: The study was registered on clinicaltrials.gov (NCT01284855) (GJ 5/1).
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Australian taipan (Oxyuranus spp.) envenoming: clinical effects and potential benefits of early antivenom therapy - Australian Snakebite Project (ASP-25).Clin Toxicol (Phila). 2017 Feb;55(2):115-122. doi: 10.1080/15563650.2016.1250903. Epub 2016 Nov 30. Clin Toxicol (Phila). 2017. PMID: 27903075
-
Death adder envenoming causes neurotoxicity not reversed by antivenom--Australian Snakebite Project (ASP-16).PLoS Negl Trop Dis. 2012;6(9):e1841. doi: 10.1371/journal.pntd.0001841. Epub 2012 Sep 27. PLoS Negl Trop Dis. 2012. PMID: 23029595 Free PMC article.
-
Clinical effects and treatment of envenoming by Hoplocephalus spp. snakes in Australia: Australian Snakebite Project (ASP-12).Toxicon. 2011 Dec 1;58(8):634-40. doi: 10.1016/j.toxicon.2011.09.013. Epub 2011 Sep 28. Toxicon. 2011. PMID: 21967812
-
Reviewing evidence of the clinical effectiveness of commercially available antivenoms in sub-Saharan Africa identifies the need for a multi-centre, multi-antivenom clinical trial.PLoS Negl Trop Dis. 2019 Jun 24;13(6):e0007551. doi: 10.1371/journal.pntd.0007551. eCollection 2019 Jun. PLoS Negl Trop Dis. 2019. PMID: 31233536 Free PMC article.
-
Situation of snakebite, antivenom market and access to antivenoms in ASEAN countries.BMJ Glob Health. 2022 Mar;7(3):e007639. doi: 10.1136/bmjgh-2021-007639. BMJ Glob Health. 2022. PMID: 35296460 Free PMC article. Review.
Cited by
-
New insights into snakebite epidemiology in Costa Rica: A retrospective evaluation of medical records.Toxicon X. 2020 Jul 30;7:100055. doi: 10.1016/j.toxcx.2020.100055. eCollection 2020 Sep. Toxicon X. 2020. PMID: 32776004 Free PMC article.
-
Analysis of snakebite data in Volta and Oti Regions, Ghana, 2019.Pan Afr Med J. 2021 Nov 3;40:131. doi: 10.11604/pamj.2021.40.131.28217. eCollection 2021. Pan Afr Med J. 2021. PMID: 34909099 Free PMC article.
-
Clinical outcomes and outcome measurement tools reported in randomised controlled trials of treatment for snakebite envenoming: A systematic review.PLoS Negl Trop Dis. 2021 Aug 2;15(8):e0009589. doi: 10.1371/journal.pntd.0009589. eCollection 2021 Aug. PLoS Negl Trop Dis. 2021. PMID: 34339410 Free PMC article.
-
Cost of Snakebite and Its Impact on Household Economy in Southern Nepal.Am J Trop Med Hyg. 2024 Nov 19;112(2):431-440. doi: 10.4269/ajtmh.24-0399. Print 2025 Feb 5. Am J Trop Med Hyg. 2024. PMID: 39561391
-
The timing is right to end snakebite deaths in South Asia.BMJ. 2019 Jan 22;364:k5317. doi: 10.1136/bmj.k5317. BMJ. 2019. PMID: 30670457 Free PMC article.
References
-
- Guidelines for the production, control and regulation of snake antivenom immunoglobulins. World Health Organisation; Geneva: 2010. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

