Two-component systems required for virulence in Pseudomonas aeruginosa
- PMID: 28510688
- PMCID: PMC5812489
- DOI: 10.1093/femsle/fnx104
Two-component systems required for virulence in Pseudomonas aeruginosa
Abstract
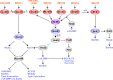
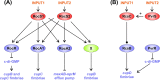
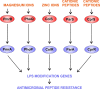
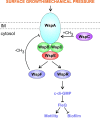
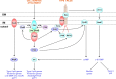
Pseudomonas aeruginosa is a versatile opportunistic pathogen capable of infecting a broad range of hosts, in addition to thriving in a broad range of environmental conditions outside of hosts. With this versatility comes the need to tightly regulate its genome to optimise its gene expression and behaviour to the prevailing conditions. Two-component systems (TCSs) comprising sensor kinases and response regulators play a major role in this regulation. This minireview discusses the growing number of TCSs that have been implicated in the virulence of P. aeruginosa, with a special focus on the emerging theme of multikinase networks, which are networks comprising multiple sensor kinases working together, sensing and integrating multiple signals to decide upon the best response. The networks covered in depth regulate processes such as the switch between acute and chronic virulence (GacS network), the Cup fimbriae (Roc network and Rcs/Pvr network), the aminoarabinose modification of lipopolysaccharide (a network involving the PhoQP and PmrBA TCSs), twitching motility and virulence (a network formed from the Chp chemosensory pathway and the FimS/AlgR TCS), and biofilm formation (Wsp chemosensory pathway). In addition, we highlight the important interfaces between these systems and secondary messenger signals such as cAMP and c-di-GMP.
Keywords: Pseudomonas aeruginosa; Two-component signalling; multikinase network; secondary messengers; virulence.
© FEMS 2017.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

