Clinical Utility of Fecal Calprotectin Monitoring in Asymptomatic Patients with Inflammatory Bowel Disease: A Systematic Review and Practical Guide
- PMID: 28511198
- PMCID: PMC5434712
- DOI: 10.1097/MIB.0000000000001082
Clinical Utility of Fecal Calprotectin Monitoring in Asymptomatic Patients with Inflammatory Bowel Disease: A Systematic Review and Practical Guide
Abstract
Background: In asymptomatic patients with inflammatory bowel disease (IBD), "monitoring" involves repeated testing aimed at early recognition of disease exacerbation. We aimed to determine the usefulness of repeated fecal calprotectin (FC) measurements to predict IBD relapses by a systematic literature review.
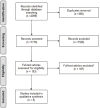
Methods: An electronic search was performed in Medline, Embase, and Cochrane from inception to April 2016. Inclusion criteria were prospective studies that followed patients with IBD in remission at baseline and had at least 2 consecutive FC measurements with a test interval of 2 weeks to 6 months. Methodological assessment was based on the second Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) checklist.
Results: A total of 1719 articles were identified; 193 were retrieved for full text review. Six studies met eligibility for inclusion. The time interval between FC tests varied between 1 and 3 months. Asymptomatic patients with IBD who had repeated FC measurements above the study's cutoff level had a 53% to 83% probability of developing disease relapse within the next 2 to 3 months. Patients with repeated normal FC values had a 67% to 94% probability to remain in remission in the next 2 to 3 months. The ideal FC cutoff for monitoring could not be identified because of the limited number studies meeting inclusion criteria and heterogeneity between selected studies.
Conclusions: Two consecutively elevated FC values are highly associated with disease relapse, indicating a consideration to proactively optimize IBD therapy plans. More prospective data are necessary to assess whether FC monitoring improves health outcomes.
Figures

Comment in
-
Clinical Utility of Fecal Calprotectin Monitoring in Asymptomatic Patients with Inflammatory Bowel Disease.Inflamm Bowel Dis. 2017 Sep;23(9):E46-E47. doi: 10.1097/MIB.0000000000001225. Inflamm Bowel Dis. 2017. PMID: 28816761 No abstract available.
-
Standardizing Fecal Calprotectin Monitoring in Asymptomatic Patients with Inflammatory Bowel Disease.Inflamm Bowel Dis. 2017 Sep;23(9):E47. doi: 10.1097/MIB.0000000000001229. Inflamm Bowel Dis. 2017. PMID: 28816762 No abstract available.
References
-
- Mant D. A framework for developing and evaluating a monitoring strategy. Evidence Based medical monitoring, from principles to practice. 2008:15–30.
-
- Peyrin-Biroulet L, Sandborn W, Sands BE, Reinisch W, Bemelman W, Bryant RV, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am J Gastroenterol. 2015;110(9):1324–38. - PubMed
-
- Papay P, Ignjatovic A, Karmiris K, Amarante H, Miheller P, Feagan B, et al. Optimising monitoring in the management of Crohn’s disease: A physician’s perspective. J Crohn’s Colitis. 2013;7(8):653–69. - PubMed
-
- Loonen HJ, Derkx BHF, Koopman HM, Heymans HSA. Are Parents Able To Rate the Symptoms and Quality of Life of Their Offspring With IBD? Inflamm Bowel Dis. 2002;8(4):270–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

