Association of Increased F4/80high Macrophages With Suppression of Serum-Transfer Arthritis in Mice With Reduced FLIP in Myeloid Cells
- PMID: 28511285
- PMCID: PMC5575965
- DOI: 10.1002/art.40151
Association of Increased F4/80high Macrophages With Suppression of Serum-Transfer Arthritis in Mice With Reduced FLIP in Myeloid Cells
Abstract
Objective: Macrophages are critical in the pathogenesis of rheumatoid arthritis (RA). We recently demonstrated that FLIP is necessary for the differentiation and/or survival of macrophages. We also showed that FLIP is highly expressed in RA synovial macrophages. This study was undertaken to determine if a reduction in FLIP in mouse macrophages reduces synovial tissue macrophages and ameliorates serum-transfer arthritis.
Methods: Mice with Flip deleted in myeloid cells (Flipf/f LysMc/+ mice) and littermate controls were used. Arthritis was induced by intraperitoneal injection of K/BxN serum. Disease severity was evaluated by clinical score and change in ankle thickness, and joints were examined by histology and immunohistochemistry. Cells were isolated from the ankles and bone marrow of the mice and examined by flow cytometry, real-time quantitative reverse transcriptase-polymerase chain reaction, or Western blotting.
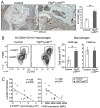
Results: In contrast to expectations, Flipf/f LysMc/+ mice developed more severe arthritis early in the clinical course, but peak arthritis was attenuated and the resolution phase more complete than in control mice. Prior to the induction of serum-transfer arthritis, the number of tissue-resident macrophages was reduced. On day 9 after arthritis induction, the number of F4/80high macrophages in the joints of the Flipf/f LysMc/+ mice was not decreased, but increased. FLIP was reduced in the F4/80high macrophages in the ankles of the Flipf/f LysMc/+ mice, while F4/80high macrophages expressed an antiinflammatory phenotype in both the Flipf/f LysMc/+ and control mice.
Conclusion: Our observations suggest that reducing FLIP in macrophages by increasing the number of antiinflammatory macrophages may be an effective therapeutic approach to suppress inflammation, depending on the disease stage.
© 2017, American College of Rheumatology.
Figures



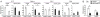

Comment in
-
Experimental arthritis: FLIPping the switch on macrophages.Nat Rev Rheumatol. 2017 Jul;13(7):390. doi: 10.1038/nrrheum.2017.90. Epub 2017 Jun 1. Nat Rev Rheumatol. 2017. PMID: 28569264 No abstract available.
Similar articles
-
Myeloid sirtuin 6 deficiency accelerates experimental rheumatoid arthritis by enhancing macrophage activation and infiltration into synovium.EBioMedicine. 2018 Dec;38:228-237. doi: 10.1016/j.ebiom.2018.11.005. Epub 2018 Nov 11. EBioMedicine. 2018. PMID: 30429089 Free PMC article.
-
NF-kappaB-regulated expression of cellular FLIP protects rheumatoid arthritis synovial fibroblasts from tumor necrosis factor alpha-mediated apoptosis.Arthritis Rheum. 2004 Dec;50(12):3844-55. doi: 10.1002/art.20680. Arthritis Rheum. 2004. PMID: 15593196
-
Differential expression pattern of the antiapoptotic proteins, Bcl-2 and FLIP, in experimental arthritis.Arthritis Rheum. 2001 Dec;44(12):2899-908. doi: 10.1002/1529-0131(200112)44:12<2899::aid-art478>3.0.co;2-x. Arthritis Rheum. 2001. PMID: 11762951
-
Expression and function of junctional adhesion molecule-C in human and experimental arthritis.Arthritis Res Ther. 2007;9(4):R65. doi: 10.1186/ar2223. Arthritis Res Ther. 2007. PMID: 17612407 Free PMC article.
-
Antiinflammatory functions of p38 in mouse models of rheumatoid arthritis: advantages of targeting upstream kinases MKK-3 or MKK-6.Arthritis Rheum. 2012 Sep;64(9):2887-95. doi: 10.1002/art.34489. Arthritis Rheum. 2012. PMID: 22488549 Free PMC article.
Cited by
-
Synovial Macrophages in Rheumatoid Arthritis: The Past, Present, and Future.Mediators Inflamm. 2020 Apr 13;2020:1583647. doi: 10.1155/2020/1583647. eCollection 2020. Mediators Inflamm. 2020. PMID: 32351318 Free PMC article. Review.
-
hnRNPK knockdown alleviates NLRP3 inflammasome priming by repressing FLIP expression in Raw264.7 macrophages.Redox Rep. 2020 Dec;25(1):104-111. doi: 10.1080/13510002.2020.1857157. Redox Rep. 2020. PMID: 33269646 Free PMC article.
-
Peptide targeting improves the delivery and therapeutic index of glucocorticoids to treat rheumatoid arthritis.J Control Release. 2024 Apr;368:329-343. doi: 10.1016/j.jconrel.2024.02.040. Epub 2024 Mar 5. J Control Release. 2024. PMID: 38431094 Free PMC article.
-
Sinomenine Inhibits the Progression of Rheumatoid Arthritis by Regulating the Secretion of Inflammatory Cytokines and Monocyte/Macrophage Subsets.Front Immunol. 2018 Sep 26;9:2228. doi: 10.3389/fimmu.2018.02228. eCollection 2018. Front Immunol. 2018. PMID: 30319663 Free PMC article. Clinical Trial.
-
Synovial Macrophages: Past Life, Current Situation, and Application in Inflammatory Arthritis.Front Immunol. 2022 Jul 26;13:905356. doi: 10.3389/fimmu.2022.905356. eCollection 2022. Front Immunol. 2022. PMID: 35958604 Free PMC article.
References
-
- McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–19. - PubMed
-
- Mulherin D, Fitzgerald O, Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum. 1996;39:115–24. - PubMed
-
- Wijbrandts CA, Vergunst CE, Haringman JJ, Gerlag DM, Smeets TJ, Tak PP. Absence of changes in the number of synovial sublining macrophages after ineffective treatment for rheumatoid arthritis: Implications for use of synovial sublining macrophages as a biomarker. Arthritis Rheum. 2007;56:3869–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

