mTOR drives cerebral blood flow and memory deficits in LDLR-/- mice modeling atherosclerosis and vascular cognitive impairment
- PMID: 28511572
- PMCID: PMC5757441
- DOI: 10.1177/0271678X17705973
mTOR drives cerebral blood flow and memory deficits in LDLR-/- mice modeling atherosclerosis and vascular cognitive impairment
Abstract
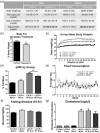
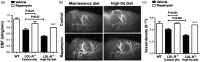
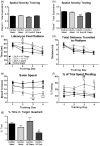
We recently showed that mTOR attenuation blocks progression and abrogates established cognitive deficits in Alzheimer's disease (AD) mouse models. These outcomes were associated with the restoration of cerebral blood flow (CBF) and brain vascular density (BVD) resulting from relief of mTOR inhibition of NO release. Recent reports suggested a role of mTOR in atherosclerosis. Because mTOR drives aging and vascular dysfunction is a universal feature of aging, we hypothesized that mTOR may contribute to brain vascular and cognitive dysfunction associated with atherosclerosis. We measured CBF, BVD, cognitive function, markers of inflammation, and parameters of cardiovascular disease in LDLR-/- mice fed maintenance or high-fat diet ± rapamycin. Cardiovascular pathologies were proportional to severity of brain vascular dysfunction. Aortic atheromas were reduced, CBF and BVD were restored, and cognitive dysfunction was attenuated potentially through reduction in systemic and brain inflammation following chronic mTOR attenuation. Our studies suggest that mTOR regulates vascular integrity and function and that mTOR attenuation may restore neurovascular function and cardiovascular health. Together with our previous studies in AD models, our data suggest mTOR-driven vascular damage may be a mechanism shared by age-associated neurological diseases. Therefore, mTOR attenuation may have promise for treatment of cognitive impairment in atherosclerosis.
Keywords: Atherosclerosis; cerebral blood flow; cognition; inflammation; vascular biology.
Figures



References
-
- Morris JC. Early-stage and preclinical Alzheimer disease. Alzheimer Dis Assoc Disord 2005; 19: 163–165. - PubMed
-
- Kuller LH, Shemanski L, Manolio T, et al. Relationship between ApoE, MRI findings, and cognitive function in the Cardiovascular Health Study. Stroke 1998; 29: 388–398. - PubMed
-
- Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol 2005; 62: 1556–1560. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

