Ethephon induced oxidative stress in the olive leaf abscission zone enables development of a selective abscission compound
- PMID: 28511694
- PMCID: PMC5434568
- DOI: 10.1186/s12870-017-1035-1
Ethephon induced oxidative stress in the olive leaf abscission zone enables development of a selective abscission compound
Abstract
Background: Table olives (Olea europaea L.), despite their widespread production, are still harvested manually. The low efficiency of manual harvesting and the rising costs of labor have reduced the profitability of this crop. A selective abscission treatment, inducing abscission of fruits but not leaves, is crucial for the adoption of mechanical harvesting of table olives. In the present work we studied the anatomical and molecular differences between the three abscission zones (AZs) of olive fruits and leaves.
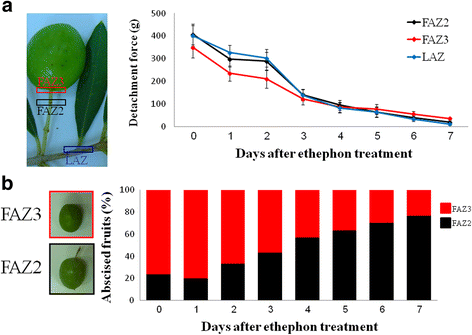
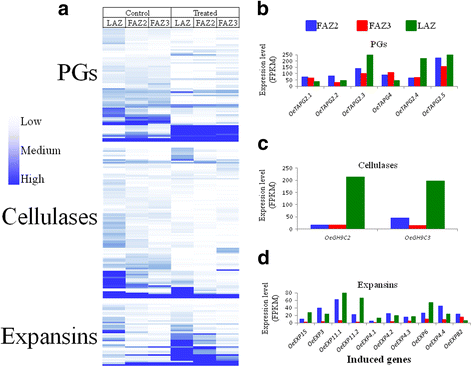
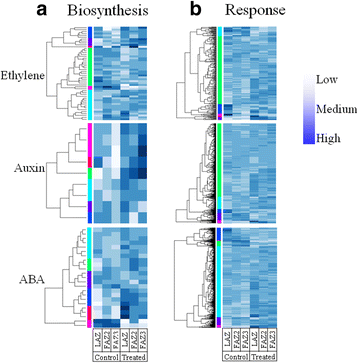
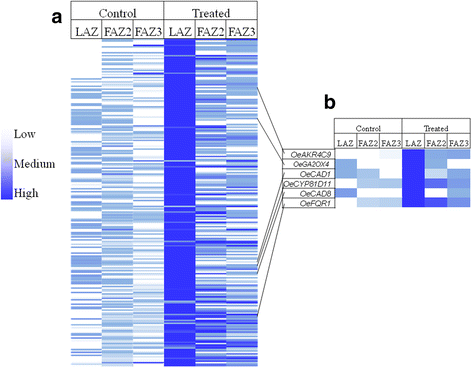
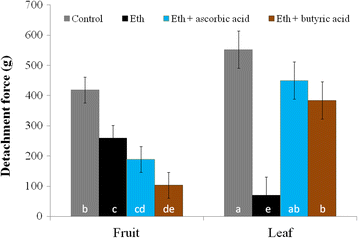
Results: The fruit abscission zone 3 (FAZ3), located between the fruit and the pedicel, was found to be the active AZ in mature fruits and is sensitive to ethephon, whereas FAZ2, between the pedicel and the rachis, is the flower active AZ as well as functioning as the most ethephon induced fruit AZ. We found anatomical differences between the leaf AZ (LAZ) and the two FAZs. Unlike the FAZs, the LAZ is characterized by small cells with less pectin compared to neighboring cells. In an attempt to differentiate between the fruit and leaf AZs, we examined the effect of treating olive-bearing trees with ethephon, an ethylene-releasing compound, with or without antioxidants, on the detachment force (DF) of fruits and leaves 5 days after the treatment. Ethephon treatment enhanced pectinase activity and reduced DF in all the three olive AZs. A transcriptomic analysis of the three olive AZs after ethephon treatment revealed induction of several genes encoding for hormones (ethylene, auxin and ABA), as well as for several cell wall degrading enzymes. However, up-regulation of cellulase genes was found only in the LAZ. Many genes involved in oxidative stress were induced by the ethephon treatment in the LAZ alone. In addition, we found that reactive oxygen species (ROS) mediated abscission in response to ethephon only in leaves. Thus, adding antioxidants such as ascorbic acid or butyric acid to the ethephon inhibited leaf abscission but enhanced fruit abscission.
Conclusion: Our findings suggest that treating olive-bearing trees with a combination of ethephon and antioxidants reduces the detachment force (DF) of fruit without weakening that of the leaves. Hence, this selective abscission treatment may be used in turn to promote mechanized harvest of olives.
Keywords: Abscission; Antioxidant; Detachment force; Ethylene; Mechanical harvesting; Olive; Oxidative stress; ROS.
Figures




References
-
- Morelló J-R, Romero M-P, Ramo T, Motilva M-J. Evaluation of l-phenylalanine ammonia-lyase activity and phenolic profile in olive drupe (Olea europaea L.) from fruit setting period to harvesting time. Plant Sci. 2005;168(1):65–72. doi: 10.1016/j.plantsci.2004.07.013. - DOI
-
- Goren R. Anatomical, Physiological, and Hormonal Aspects of Abscission in Citrus, in Horticultural Reviews, Volume 15 (ed J. Janick). Oxford: Wiley; 1993. doi:10.1002/9780470650547.ch4.
-
- Biain de Elizalde MM. Histology of the abscission zones of tomato flowers and fruit and some effects of 2-chloroethylphosphonic acid (Ethrel) application. Phyton. 1980;38:71–79.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

