A structure-derived snap-trap mechanism of a multispecific serpin from the dysbiotic human oral microbiome
- PMID: 28512127
- PMCID: PMC5491774
- DOI: 10.1074/jbc.M117.786533
A structure-derived snap-trap mechanism of a multispecific serpin from the dysbiotic human oral microbiome
Abstract
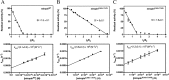
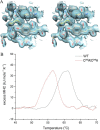
Enduring host-microbiome relationships are based on adaptive strategies within a particular ecological niche. Tannerella forsythia is a dysbiotic member of the human oral microbiome that inhabits periodontal pockets and contributes to chronic periodontitis. To counteract endopeptidases from the host or microbial competitors, T. forsythia possesses a serpin-type proteinase inhibitor called miropin. Although serpins from animals, plants, and viruses have been widely studied, those from prokaryotes have received only limited attention. Here we show that miropin uses the serpin-type suicidal mechanism. We found that, similar to a snap trap, the protein transits from a metastable native form to a relaxed triggered or induced form after cleavage of a reactive-site target bond in an exposed reactive-center loop. The prey peptidase becomes covalently attached to the inhibitor, is dragged 75 Å apart, and is irreversibly inhibited. This coincides with a large conformational rearrangement of miropin, which inserts the segment upstream of the cleavage site as an extra β-strand in a central β-sheet. Standard serpins possess a single target bond and inhibit selected endopeptidases of particular specificity and class. In contrast, miropin uniquely blocked many serine and cysteine endopeptidases of disparate architecture and substrate specificity owing to several potential target bonds within the reactive-center loop and to plasticity in accommodating extra β-strands of variable length. Phylogenetic studies revealed a patchy distribution of bacterial serpins incompatible with a vertical descent model. This finding suggests that miropin was acquired from the host through horizontal gene transfer, perhaps facilitated by the long and intimate association of T. forsythia with the human gingiva.
Keywords: inhibition mechanism; inhibitor; molecular biology; peptidase; periodontal disease; protease; protease inhibitor; protein structure; proteinase; proteolysis.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

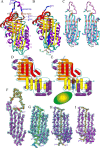

References
-
- Leevvenhoeck A. (1683) An abstract of a letter from Mr. Anthony Leevvenhoeck at Delft, dated Sep. 17. 1683, containing some microscopical observations, about animals in the scurf of the teeth, the substance call'd worms in the nose, the cuticula consisting of scales. Philos. Trans. 14, 568–574
-
- Kuo L. C., Polson A. M., and Kang T. (2008) Associations between periodontal diseases and systemic diseases: a review of the inter-relationships and interactions with diabetes, respiratory diseases, cardiovascular diseases and osteoporosis. Public Health 122, 417–433 - PubMed
-
- Warinner C., Rodrigues J. F., Vyas R., Trachsel C., Shved N., Grossmann J., Radini A., Hancock Y., Tito R. Y., Fiddyment S., Speller C., Hendy J., Charlton S., Luder H. U., Salazar-García D. C., et al. (2014) Pathogens and host immunity in the ancient human oral cavity. Nat. Genet. 46, 336–344 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

