Hematopoietic origin of Langerhans cell histiocytosis and Erdheim-Chester disease in adults
- PMID: 28512190
- PMCID: PMC5524529
- DOI: 10.1182/blood-2016-12-757823
Hematopoietic origin of Langerhans cell histiocytosis and Erdheim-Chester disease in adults
Abstract
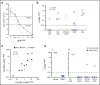
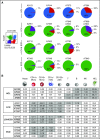
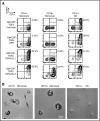
Langerhans cell histiocytosis (LCH) and Erdheim-Chester disease (ECD) are rare histiocytic disorders induced by somatic mutation of MAPK pathway genes. BRAFV600E mutation is the most common mutation in both conditions and also occurs in the hematopoietic neoplasm hairy cell leukemia (HCL). It is not known if adult LCH or ECD arises from hematopoietic stem cells (HSCs), nor which potential blood borne precursors lead to the formation of histiocytic lesions. In this study, BRAFV600E allele-specific polymerase chain reaction was used to map the neoplastic clone in 20 adults with LCH, ECD, and HCL. BRAFV600E was tracked to classical monocytes, nonclassical monocytes, and CD1c+ myeloid dendritic cells (DCs) in the blood, and mutations were observed in HSCs and myeloid progenitors in the bone marrow of 4 patients. The pattern of involvement of peripheral blood myeloid cells was indistinguishable between LCH and ECD, although the histiocytic disorders were distinct to HCL. As reported in children, detection of BRAFV600E in peripheral blood of adults was a marker of active multisystem LCH. The healthy counterparts of myeloid cells affected by BRAF mutation had a range of differentiation potentials depending on exogenous signals. CD1c+ DCs acquired high langerin and CD1a with granulocyte-macrophage colony-stimulating factor and transforming growth factor β alone, whereas CD14+ classical monocytes required additional notch ligation. Both classical and nonclassical monocytes, but not CD1c+ DCs, made foamy macrophages easily in vitro with macrophage colony-stimulating factor and human serum. These studies are consistent with a hematopoietic origin and >1 immediate cellular precursor in both LCH and ECD.
© 2017 by The American Society of Hematology.
Figures



References
-
- Sahm F, Capper D, Preusser M, et al. BRAFV600E mutant protein is expressed in cells of variable maturation in Langerhans cell histiocytosis. Blood. 2012;120(12):e28-e34. - PubMed
-
- Haroche J, Charlotte F, Arnaud L, et al. High prevalence of BRAF V600E mutations in Erdheim-Chester disease but not in other non-Langerhans cell histiocytoses. Blood. 2012;120(13):2700-2703. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

