Loss of the homologous recombination gene rad51 leads to Fanconi anemia-like symptoms in zebrafish
- PMID: 28512217
- PMCID: PMC5465903
- DOI: 10.1073/pnas.1620631114
Loss of the homologous recombination gene rad51 leads to Fanconi anemia-like symptoms in zebrafish
Abstract
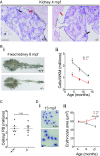
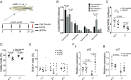
RAD51 is an indispensable homologous recombination protein, necessary for strand invasion and crossing over. It has recently been designated as a Fanconi anemia (FA) gene, following the discovery of two patients carrying dominant-negative mutations. FA is a hereditary DNA-repair disorder characterized by various congenital abnormalities, progressive bone marrow failure, and cancer predisposition. In this report, we describe a viable vertebrate model of RAD51 loss. Zebrafish rad51 loss-of-function mutants developed key features of FA, including hypocellular kidney marrow, sensitivity to cross-linking agents, and decreased size. We show that some of these symptoms stem from both decreased proliferation and increased apoptosis of embryonic hematopoietic stem and progenitor cells. Comutation of p53 was able to rescue the hematopoietic defects seen in the single mutants, but led to tumor development. We further demonstrate that prolonged inflammatory stress can exacerbate the hematological impairment, leading to an additional decrease in kidney marrow cell numbers. These findings strengthen the assignment of RAD51 as a Fanconi gene and provide more evidence for the notion that aberrant p53 signaling during embryogenesis leads to the hematological defects seen later in life in FA. Further research on this zebrafish FA model will lead to a deeper understanding of the molecular basis of bone marrow failure in FA and the cellular role of RAD51.
Keywords: Fanconi anemia; cytokine effects; hematopoiesis; inflammation; stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

