Immune consequences of penfluridol treatment associated with inhibition of glioblastoma tumor growth
- PMID: 28512255
- PMCID: PMC5564593
- DOI: 10.18632/oncotarget.17425
Immune consequences of penfluridol treatment associated with inhibition of glioblastoma tumor growth
Abstract
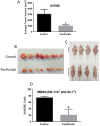
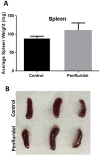
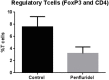
Glioblastoma is the most common and lethal brain tumor associated with only 12% median survival rate of patients. Despite the development of advanced surgical, radiation or use of combinations of anti-cancer drugs, treatment for glioblastoma patients is still a challenge. The major contributing factor in glioblastoma progression and resistive nature is its ability to evade the immune surveillance. Hence, modulating the immune system in glioblastoma tumors could be an important strategy for anticancer therapeutics. Penfluridol, an antipsychotic drug has been shown to have anti-cancer properties in our recently published studies. The present study evaluates the immune response of penfluridol in glioblastoma tumors. Our results demonstrated that penfluridol treatment significantly suppressed glioblastoma tumor growth. Our current results demonstrated about 72% suppression of myeloid derived suppressor cells (MDSCs) with penfluridol treatment in mouse bearing U87MG glioblastoma tumors. MDSCs are known to increase regulatory T cells (Treg), which are immunosuppressive in nature and suppresses M1 macrophages that are tumor suppressive in nature. Our results also showed suppression of regulatory T cells as well as elevation of M1 macrophages with penfluridol treatment by 58% and 57% respectively. Decrease in CCL4 as well as IFNγ with penfluridol treatment was also observed indicating decrease in overall tumor inflammation. This is the first report demonstrating immune modulations by penfluridol treatment associated with glioblastoma tumor growth suppression prompting further investigation to establish penfluridol as a treatment option for glioblastoma patients.
Keywords: MDSC; Treg; anti-psychotic drug; glioblastoma; macrophages.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Kohanbash G, Okada H. Myeloid-derived suppressor cells (MDSCs) in gliomas and glioma-development. Immunol Invest. 2012;41:658–679. - PubMed
-
- Thaci B, Ahmed AU, Ulasov IV, Wainwright DA, Nigam P, Auffinger B, Tobias AL, Han Y, Zhang L, Moon KS, Lesniak MS. Depletion of myeloid-derived suppressor cells during interleukin-12 immunogene therapy does not confer a survival advantage in experimental malignant glioma. Cancer Gene Ther. 2014;21:38–44. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

