Phenotypic plasticity, trade-offs and gene expression changes accompanying dietary restriction and switches in Bactrocera dorsalis (Hendel) (Diptera: Tephritidae)
- PMID: 28512316
- PMCID: PMC5434071
- DOI: 10.1038/s41598-017-02106-3
Phenotypic plasticity, trade-offs and gene expression changes accompanying dietary restriction and switches in Bactrocera dorsalis (Hendel) (Diptera: Tephritidae)
Abstract
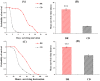
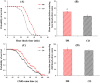
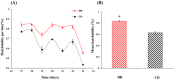
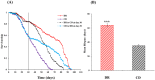
In this study, we investigated the effects of dietary restriction (DR) and variable diets on phenotypes and gene expression in oriental fruit fly, Bactrocera dorsalis (Hendel), one of the most economically important pests in the family Tephritidae around the world. As expected, we found that DR altered the B. dorsalis phenotypes by significantly increasing stress resistance and lifespan, but reduced egg production when compared with the control diet. The results suggested a trade-off between reproduction versus somatic maintenance (stress resistance) and lifespan in B. dorsalis. Diet also had a significant effect on hatchability, and DR could increase the egg hatching success of B. dorsalis. Furthermore, DR up-regulated metabolic pathways involved in energy homeostasis and down-regulated pathways in egg production, which might mediate trade-offs between somatic maintenance and reproduction under DR regimes. The gene expression profiles in response to the acute dietary switches indicated that the digestive and metabolic pathways maybe involved in the adaptability of flies to variable dietary resources. In summary, the research facilitates a better understanding of the molecular mechanisms responsible for the B. dorsalis' phenotypic adjustments to the different qualities of the available diets.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Phenotypes, antioxidant responses, and gene expression changes accompanying a sugar-only diet in Bactrocera dorsalis (Hendel) (Diptera: Tephritidae).BMC Evol Biol. 2017 Aug 17;17(1):194. doi: 10.1186/s12862-017-1045-5. BMC Evol Biol. 2017. PMID: 28814277 Free PMC article.
-
The effect of dietary restriction on longevity, fecundity, and antioxidant responses in the oriental fruit fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae).J Insect Physiol. 2013 Oct;59(10):1008-16. doi: 10.1016/j.jinsphys.2013.07.006. Epub 2013 Aug 1. J Insect Physiol. 2013. PMID: 23911350
-
Characterizing the developmental transcriptome of the oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae) through comparative genomic analysis with Drosophila melanogaster utilizing modENCODE datasets.BMC Genomics. 2014 Oct 28;15(1):942. doi: 10.1186/1471-2164-15-942. BMC Genomics. 2014. PMID: 25348373 Free PMC article.
-
Phytosanitary Treatments Against Bactrocera dorsalis (Diptera: Tephritidae): Current Situation and Future Prospects.J Econ Entomol. 2017 Feb 1;110(1):67-79. doi: 10.1093/jee/tow247. J Econ Entomol. 2017. PMID: 28028169 Review.
-
Recent research status of Bactrocera dorsalis: Insights from resistance mechanisms and population structure.Arch Insect Biochem Physiol. 2019 Nov;102(3):e21601. doi: 10.1002/arch.21601. Epub 2019 Jul 22. Arch Insect Biochem Physiol. 2019. PMID: 31328817 Review.
Cited by
-
Dietary restriction reveals sex-specific expression of the mTOR pathway genes in Japanese quails.Sci Rep. 2024 Apr 9;14(1):8314. doi: 10.1038/s41598-024-58487-9. Sci Rep. 2024. PMID: 38594358 Free PMC article.
-
Genetic and Ecological Relationships of Anastrepha ludens (Diptera: Tephritidae) Populations in Southern Mexico.Insects. 2020 Nov 19;11(11):815. doi: 10.3390/insects11110815. Insects. 2020. PMID: 33227892 Free PMC article.
-
Feeding on the Fruit Waste Orange Bagasse Modifies Immature Protein Content, Body Weight, Scent Bouquet Composition, and Copula Duration in Males of a Tephritid Frugivorous Fly.Biology (Basel). 2023 May 19;12(5):739. doi: 10.3390/biology12050739. Biology (Basel). 2023. PMID: 37237551 Free PMC article.
-
Dietary-based developmental plasticity affects juvenile survival in an aquatic detritivore.Proc Biol Sci. 2021 Feb 24;288(1945):20203136. doi: 10.1098/rspb.2020.3136. Epub 2021 Feb 17. Proc Biol Sci. 2021. PMID: 33593189 Free PMC article.
-
Eco-evolutionary causes and consequences of temporal changes in intratumoural blood flow.Nat Rev Cancer. 2018 Sep;18(9):576-585. doi: 10.1038/s41568-018-0030-7. Nat Rev Cancer. 2018. PMID: 29891961 Free PMC article. Review.
References
-
- Roff, D. A. Life history evolution. Sinauer Associates Inc., Sunderland (2002).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

