Salinity Stress Does Not Affect Root Uptake, Dissemination and Persistence of Salmonella in Sweet-basil (Ocimum basilicum)
- PMID: 28512466
- PMCID: PMC5411819
- DOI: 10.3389/fpls.2017.00675
Salinity Stress Does Not Affect Root Uptake, Dissemination and Persistence of Salmonella in Sweet-basil (Ocimum basilicum)
Abstract
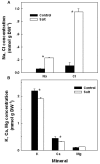
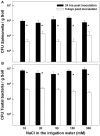
Crop produce can be contaminated in the field during cultivation by bacterial human pathogens originating from contaminated soil or irrigation water. The bacterial pathogens interact with the plant, can penetrate the plant via the root system and translocate and survive in above-ground tissues. The present study is first to investigate effects of an abiotic stress, salinity, on the interaction of plants with a bacterial human pathogen. The main sources of human bacterial contamination of plants are manures and marginal irrigation waters such as treated or un-treated wastewater. These are often saline and induce morphological, chemical and physiological changes in plants that might affect the interaction between the pathogens and the plant and thereby the potential for plant contamination. This research studied effects of salinity on the internalization of the bacterial human pathogen Salmonella enterica serovar Newport via the root system of sweet-basil plants, dissemination of the bacteria in the plant, and kinetics of survival in planta. Irrigation with 30 mM NaCl-salinity induced typical salt-stress effects on the plant: growth was reduced, Na and Cl concentrations increased, K and Ca concentrations reduced, osmotic potential and anti-oxidative activity were increased by 30%, stomatal conductance was reduced, and concentrations of essential-oils in the plants increased by 26%. Despite these physical, chemical and morphological changes in the plants, root internalization of the bacteria and its translocation to the shoot were not affected, and neither was the die-off rate of Salmonella in planta. The results demonstrate that the salinity-induced changes in the sweet-basil plants did not affect the interaction between Salmonella and the plant and thereby the potential for crop contamination.
Keywords: Salmonella; basil; contamination; human pathogens; internalization; persistence; root; salt stress.
Figures




References
-
- Archer D. L. (1996). Preservation microbiology and safety: evidence that stress enhances virulence, and triggers adaptive mutations. Trends Food Sci. Technol. 7 91–95. 10.1016/0924-2244(96)81303-3 - DOI
-
- Bagamboula C. F., Uyttendaeleand M., Debevere J. (2004), Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. flexneri Food Microbiol. 21 33–42. 10.1016/S0740-0020(03)00046-7 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

