MRI in Glioma Immunotherapy: Evidence, Pitfalls, and Perspectives
- PMID: 28512646
- PMCID: PMC5415864
- DOI: 10.1155/2017/5813951
MRI in Glioma Immunotherapy: Evidence, Pitfalls, and Perspectives
Abstract
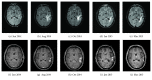
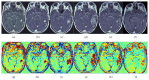
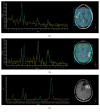
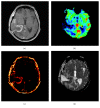
Pseudophenomena, that is, imaging alterations due to therapy rather than tumor evolution, have an important impact on the management of glioma patients and the results of clinical trials. RANO (response assessment in neurooncology) criteria, including conventional MRI (cMRI), addressed the issues of pseudoprogression after radiotherapy and concomitant chemotherapy and pseudoresponse during antiangiogenic therapy of glioblastomas (GBM) and other gliomas. The development of cancer immunotherapy forced the identification of further relevant response criteria, summarized by the iRANO working group in 2015. In spite of this, the unequivocal definition of glioma progression by cMRI remains difficult particularly in the setting of immunotherapy approaches provided by checkpoint inhibitors and dendritic cells. Advanced MRI (aMRI) may in principle address this unmet clinical need. Here, we discuss the potential contribution of different aMRI techniques and their indications and pitfalls in relation to biological and imaging features of glioma and immune system interactions.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

