Peptide-Functionalized Fluorescent Particles for In Situ Detection of Nitric Oxide via Peroxynitrite-Mediated Nitration
- PMID: 28512791
- PMCID: PMC5568941
- DOI: 10.1002/adhm.201700383
Peptide-Functionalized Fluorescent Particles for In Situ Detection of Nitric Oxide via Peroxynitrite-Mediated Nitration
Abstract
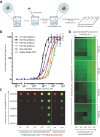
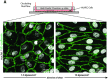
Nitric oxide (NO) is a free radical signaling molecule that plays a crucial role in modulating physiological homeostasis across multiple biological systems. NO dysregulation is linked to the pathogenesis of multiple diseases; therefore, its quantification is important for understanding pathophysiological processes. The detection of NO is challenging, typically limited by its reactive nature and short half-life. Additionally, the presence of interfering analytes and accessibility to biological fluids in the native tissues make the measurement technically challenging and often unreliable. Here, a bio-inspired peptide-based NO sensor is developed, which detects NO-derived oxidants, predominately peroxynitrite-mediated nitration of tyrosine residues. It is demonstrated that these peptide-based NO sensors can detect peroxynitrite-mediated nitration in response to physiological shear stress by endothelial cells in vitro. Using the peptide-conjugated fluorescent particle immunoassay, peroxynitrite-mediated nitration activity with a detection limit of ≈100 × 10-9 m is detected. This study envisions that the NO detection platform can be applied to a multitude of applications including monitoring of NO activity in healthy and diseased tissues, localized detection of NO production of specific cells, and cell-based/therapeutic screening of peroxynitrite levels to monitor pronitroxidative stress in biological samples.
Keywords: endothelial cells; immunoassays; nitric oxide detection; peptide biosensors; peroxynitrite.
© 2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

