Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways
- PMID: 28513565
- PMCID: PMC5447962
- DOI: 10.3390/cancers9050052
Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways
Abstract
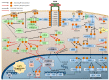
The epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase that is commonly upregulated in cancers such as in non-small-cell lung cancer, metastatic colorectal cancer, glioblastoma, head and neck cancer, pancreatic cancer, and breast cancer. Various mechanisms mediate the upregulation of EGFR activity, including common mutations and truncations to its extracellular domain, such as in the EGFRvIII truncations, as well as to its kinase domain, such as the L858R and T790M mutations, or the exon 19 truncation. These EGFR aberrations over-activate downstream pro-oncogenic signaling pathways, including the RAS-RAF-MEK-ERK MAPK and AKT-PI3K-mTOR pathways. These pathways then activate many biological outputs that are beneficial to cancer cell proliferation, including their chronic initiation and progression through the cell cycle. Here, we review the molecular mechanisms that regulate EGFR signal transduction, including the EGFR structure and its mutations, ligand binding and EGFR dimerization, as well as the signaling pathways that lead to G1 cell cycle progression. We focus on the induction of CYCLIN D expression, CDK4/6 activation, and the repression of cyclin-dependent kinase inhibitor proteins (CDKi) by EGFR signaling pathways. We also discuss the successes and challenges of EGFR-targeted therapies, and the potential for their use in combination with CDK4/6 inhibitors.
Keywords: AKT; ERK; ErBB; G1; cell cycle; cell proliferation; epidermal growth factor receptor; signal transduction.
Conflict of interest statement
There are no conflicts of interest to report.
Figures
References
-
- Cohen S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J. Biol. Chem. 1962;237:1555–1562. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

