PPARγ Agonists Attenuate Trigeminal Neuropathic Pain
- PMID: 28514232
- PMCID: PMC5675763
- DOI: 10.1097/AJP.0000000000000509
PPARγ Agonists Attenuate Trigeminal Neuropathic Pain
Abstract
Objectives: The aim of this study is to investigate the role of peroxisome proliferator-activated receptor-gamma isoform (PPARγ), in trigeminal neuropathic pain utilizing a novel mouse trigeminal inflammatory compression (TIC) injury model.
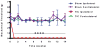
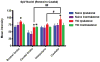
Results: The study determined that the PPARγ nuclear receptor plays a significant role in trigeminal nociception transmission, evidenced by: 1) Intense PPARγ immunoreactivity is expressed 3 weeks after TIC nerve injury in the spinal trigeminal caudalis, the termination site of trigeminal nociceptive nerve fibers. 2) Systemic administration of a PPARγ agonist, pioglitazone (PIO), attenuates whisker pad mechanical allodynia at doses of 300 mg/kg i.p. and 600 mg/kg p.o. 3) Administration of a PPARγ antagonist, GW9662 (30 mg/kg i.p.), prior to providing the optimal dose of PIO (300 mg/kg i.p.) blocked the analgesic effect of PIO.
Discussion: This is the first study localizing PPARγ immunoreactivity throughout the brainstem trigeminal sensory spinal nucleus (spV) and its increase three weeks after TIC nerve injury. This is also the first study to demonstrate that activation of PPARγ attenuates trigeminal hypersensitivity in the mouse TIC nerve injury model. The findings presented here suggest the possibility of utilizing the FDA approved diabetic treatment drug, PIO, as a new therapeutic that targets PPARγ for treatment of patients suffering from orofacial neuropathic pain.
Figures



References
-
- Zakrzewska JM. Differential diagnosis of facial pain and guidelines for management. Br J Anaesth. 2013;111:95–104. - PubMed
-
- Asmundson GJ, Katz J. Understanding the co-occurrence of anxiety disorders and chronic pain: state-of-the-art. Depress Anxiety. 2009;26:888–901. - PubMed
-
- De Leeuw R. Orofacial Pain: Guidelines for Assessment, Diagnosis, and Management. 4. Hanover Park, IL: Quintessence Publishing Co, Inc; 2008. p. 316.
-
- Robinson MJ, Edwards SE, Iyengar S, Bymaster F, Clark M, Katon W. Depression and pain. Front Biosci (Landmark Ed) 2009;14:5031–51. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

