PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity
- PMID: 28514441
- PMCID: PMC5931375
- DOI: 10.1038/nature22396
PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity
Abstract
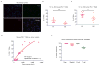
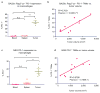
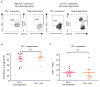
Programmed cell death protein 1 (PD-1) is an immune checkpoint receptor that is upregulated on activated T cells for the induction of immune tolerance. Tumour cells frequently overexpress the ligand for PD-1, programmed cell death ligand 1 (PD-L1), facilitating their escape from the immune system. Monoclonal antibodies that block the interaction between PD-1 and PD-L1, by binding to either the ligand or receptor, have shown notable clinical efficacy in patients with a variety of cancers, including melanoma, colorectal cancer, non-small-cell lung cancer and Hodgkin's lymphoma. Although it is well established that PD-1-PD-L1 blockade activates T cells, little is known about the role that this pathway may have in tumour-associated macrophages (TAMs). Here we show that both mouse and human TAMs express PD-1. TAM PD-1 expression increases over time in mouse models of cancer and with increasing disease stage in primary human cancers. TAM PD-1 expression correlates negatively with phagocytic potency against tumour cells, and blockade of PD-1-PD-L1 in vivo increases macrophage phagocytosis, reduces tumour growth and lengthens the survival of mice in mouse models of cancer in a macrophage-dependent fashion. This suggests that PD-1-PD-L1 therapies may also function through a direct effect on macrophages, with substantial implications for the treatment of cancer with these agents.
Figures


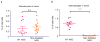


Comment in
-
Immunotherapy: Exploiting PD-1 on TAMs for tumour cell kill.Nat Rev Clin Oncol. 2017 Jul;14(7):392-393. doi: 10.1038/nrclinonc.2017.87. Epub 2017 Jun 13. Nat Rev Clin Oncol. 2017. PMID: 28607517 No abstract available.
-
Immunotherapy: Macrophages steal the show.Nat Rev Cancer. 2017 Jul 23;17(7):396-397. doi: 10.1038/nrc.2017.47. Nat Rev Cancer. 2017. PMID: 28642605 No abstract available.
-
Cancer immunotherapy: Macrophages steal the show.Nat Rev Drug Discov. 2017 Jun 29;16(7):455. doi: 10.1038/nrd.2017.126. Nat Rev Drug Discov. 2017. PMID: 28660900 No abstract available.
-
Tumor-associated macrophages-additional effectors at anti-PD-1/PD-L1 therapy?J Thorac Dis. 2017 Nov;9(11):4197-4200. doi: 10.21037/jtd.2017.10.15. J Thorac Dis. 2017. PMID: 29268470 Free PMC article. No abstract available.
-
Why anti-PD1/PDL1 therapy is so effective? Another piece in the puzzle.J Thorac Dis. 2017 Dec;9(12):4863-4866. doi: 10.21037/jtd.2017.11.105. J Thorac Dis. 2017. PMID: 29312678 Free PMC article. No abstract available.
References
-
- Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

