Identification of preoptic sleep neurons using retrograde labelling and gene profiling
- PMID: 28514446
- PMCID: PMC5554302
- DOI: 10.1038/nature22350
Identification of preoptic sleep neurons using retrograde labelling and gene profiling
Abstract
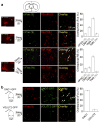
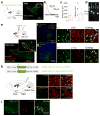
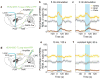
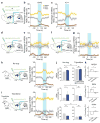
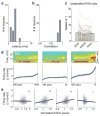
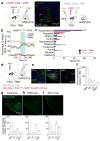
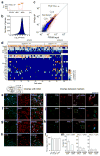
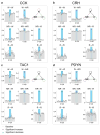
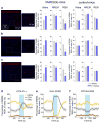
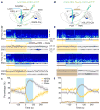
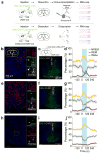
In humans and other mammalian species, lesions in the preoptic area of the hypothalamus cause profound sleep impairment, indicating a crucial role of the preoptic area in sleep generation. However, the underlying circuit mechanism remains poorly understood. Electrophysiological recordings and c-Fos immunohistochemistry have shown the existence of sleep-active neurons in the preoptic area, especially in the ventrolateral preoptic area and median preoptic nucleus. Pharmacogenetic activation of c-Fos-labelled sleep-active neurons has been shown to induce sleep. However, the sleep-active neurons are spatially intermingled with wake-active neurons, making it difficult to target the sleep neurons specifically for circuit analysis. Here we identify a population of preoptic area sleep neurons on the basis of their projection target and discover their molecular markers. Using a lentivirus expressing channelrhodopsin-2 or a light-activated chloride channel for retrograde labelling, bidirectional optogenetic manipulation, and optrode recording, we show that the preoptic area GABAergic neurons projecting to the tuberomammillary nucleus are both sleep active and sleep promoting. Furthermore, translating ribosome affinity purification and single-cell RNA sequencing identify candidate markers for these neurons, and optogenetic and pharmacogenetic manipulations demonstrate that several peptide markers (cholecystokinin, corticotropin-releasing hormone, and tachykinin 1) label sleep-promoting neurons. Together, these findings provide easy genetic access to sleep-promoting preoptic area neurons and a valuable entry point for dissecting the sleep control circuit.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Von Economo C. Sleep as a problem of localization. J Nerv Ment Dis. 1930;71:249–259.
-
- Nauta WJ. Hypothalamic regulation of sleep in rats; an experimental study. J Neurophysiol. 1946;9:285–316. - PubMed
-
- McGinty DJ, Sterman MB. Sleep suppression after basal forebrain lesions in the cat. Science. 1968;160:1253–1255. - PubMed
-
- Sallanon M, et al. Long-lasting insomnia induced by preoptic neuron lesions and its transient reversal by muscimol injection into the posterior hypothalamus in the cat. Neuroscience. 1989;32:669–683. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

