Methadone for neuropathic pain in adults
- PMID: 28514508
- PMCID: PMC6353163
- DOI: 10.1002/14651858.CD012499.pub2
Methadone for neuropathic pain in adults
Abstract
Background: This review replaces an earlier review, "Methadone for chronic non-cancer pain in adults". This review serves to update the original and includes only studies of neuropathic pain. Methadone belongs to a class of analgesics known as opioids, that are considered the cornerstone of therapy for moderate-to-severe postsurgical pain and pain due to life-threatening illnesses; however, their use in neuropathic pain is controversial. Methadone has many characteristics that differentiate it from other opioids, which suggests that it may have a different efficacy and safety profile.
Objectives: To assess the analgesic efficacy and adverse events of methadone for chronic neuropathic pain in adults.
Search methods: We searched the following databases: CENTRAL (CRSO), MEDLINE (Ovid), and Embase (Ovid), and two clinical trial registries. We also searched the reference lists of retrieved articles. The date of the most recent search was 30 November 2016.
Selection criteria: We included randomised, double-blind studies of two weeks' duration or longer, comparing methadone (in any dose, administered by any route, and in any formulation) with placebo or another active treatment in chronic neuropathic pain.
Data collection and analysis: We used standard methodological procedures expected by Cochrane. Two review authors independently considered trials for inclusion in the review, assessed risk of bias, and extracted data. There were insufficient data to perform pooled analyses. We assessed the overall quality of the evidence for each outcome using GRADE and created a 'Summary of findings' table.
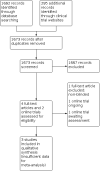
Main results: We included three studies, involving 105 participants. All were cross-over studies, one involving 19 participants with diverse neuropathic pain syndromes, the other two involving 86 participants with postherpetic neuralgia. Study phases ranged from 20 days to approximately eight weeks. All administered methadone orally, in doses ranging from 10 mg to 80 mg daily. Comparators were primarily placebo, but one study also included morphine and tricyclic antidepressants.The included studies had several limitations related to risk of bias, particularly incomplete reporting, selective outcome reporting, and small sample sizes.There were very limited data for our primary outcomes of participants with at least 30% or at least 50% pain relief. Two studies reported that 11/29 participants receiving methadone achieved 30% pain relief versus 7/29 participants receiving placebo. Only one study presented data in a manner that allowed us to calculate the number of participants with at least 50% pain relief. None of the 19 participants achieved a 50% reduction in pain intensity, either when receiving methadone or when receiving placebo. No study provided data for our other primary outcomes of Patient Global Impression of Change scale (PGIC) much or very much improved (equivalent to at least 30% pain relief) and PGIC very much improved (equivalent to at least 50% pain relief).For secondary efficacy outcomes, one study reported maximum and mean pain intensity and pain relief, and reported statistically significant improvements versus placebo for all outcomes with 20 mg daily doses of methadone, but not with 10 mg daily doses. The second study reported differences in pain reduction between methadone (n = 26) and morphine (n = 38) and found morphine to be statistically superior. The third study reported the number of responders (variously defined) for several pain and functional outcomes and found methadone to be statistically superior to placebo for the outcomes of categorical pain intensity and evoked pain. In the two studies that reported data, 0/29 participants withdrew due to lack of efficacy, whereas 4/29 participants withdrew due to adverse events while taking methadone versus 3/29 while taking placebo.One study reported incidences for several individual adverse events, but found a statistically significant increased incidence for methadone over placebo for only one event, dizziness. The other studies did not report data in a manner that enabled us to analyze adverse events. There were no serious adverse events or deaths reported.We assessed the quality of the evidence as very low for all efficacy and safety outcomes using GRADE, primarily because of the heterogeneity of study designs and populations, short durations, cross-over methodology, and few participants and events.
Authors' conclusions: The three studies provide very limited, very low quality evidence of the efficacy and safety of methadone for chronic neuropathic pain, and there were too few data for pooled analysis of efficacy or harm, or to have confidence in the results of the individual studies. No conclusions can be made regarding differences in efficacy or safety between methadone and placebo, other opioids, or other treatments.
Conflict of interest statement
EM: none known; EM is a pharmacist with a Master's degree in Pain Research, Education and Policy, and is involved in the pharmacological management of patients with chronic pain issues in a tertiary setting.
MF: none known.
RS: none known; RS is an anaesthesiologist with experience and expertise in acute pain management, including managing patients with chronic pain undergoing surgical procedures.
Figures
Update of
References
References to studies included in this review
Morley 2003 {published data only}
Raja 2002 {published data only}
References to studies excluded from this review
Haumann 2016 {published data only}
References to studies awaiting assessment
NCT02233452 {published data only}
-
- NCT02233452. Management of Neuropathic Chronic Pain with Methadone Combined with Ketamine: Randomized, Double Blind, Active-controlled Clinical Trial. clinicaltrials.gov/ct2/show/NCT02233452 Date first received: 22 August 2014.
References to ongoing studies
NCT01205516 {published data only}
-
- NCT01205516. Methadone in Neuropathic Pain. clinicaltrials.gov/ct2/show/NCT01205516 Date first received: 17 September 2010.
Additional references
Baron 2012
Berger 2004
Berger 2009
Berger 2012
Bouhassira 2008
Bruera 1991
Bruera 2002
Calvo 2012
CDC 2012
-
- Centers for Disease Control and Prevention. Prescription painkiller overdoses: use and abuse of methadone as a painkiller, 2012. www.cdc.gov/vitalsigns/MethadoneOverdoses/index.html (accessed 16 June 2016).
CDC 2014
-
- Centers for Disease Control and Prevention. Opioid painkiller prescribing. www.cdc.gov/vitalsigns/opioid-prescribing/index.html (accessed 17 June 2016).
Chugh 2008
Davis 2001
Dechartres 2013
Dechartres 2014
Demant 2014
-
- Demant DT, Lund K, Vollert J, Maier C, Segerdahl M, Finnerup NB, et al. The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: a randomised, double-blind, placebo-controlled phenotype-stratified study. Pain 2014;155(11):2263-73. [DOI: 10.1016/j.pain.2014.08.014] - DOI - PubMed
Derry 2012
Derry 2014
Derry 2017
Dworkin 2008
Dworkin 2013
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta-analysis involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31:140-9. - PubMed
Fainsinger 1993
Finnerup 2013
Finnerup 2015
Galer 1997
-
- Galer BS, Jensen MP. Development and preliminary evaluation of a pain measure specific to neuropathic pain: the Neuropathic Pain Scale. Neurology 1997;48:332-8. - PubMed
Gannon 1997
-
- Gannon C. The use of methadone in the care of the dying. European Journal of Palliative Care 1997;4:152-8.
Gaskell 2016
Gustorff 2008
Guyatt 2013a
-
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso-Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66:151-7. [DOI: 10.1016/j.jclinepi.2012.01.006] - DOI - PubMed
Guyatt 2013b
Hall 2008
Helfert 2015
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hoffman 2010
Jadad 1996
Jensen 2011
Kalso 2013
Katusic 1991
Koopman 2009
Krantz 2009
-
- Krantz MJ, Martin J, Stimmel B, Mehta D, Haigney MC. QTc interval screening in methadone treatment. Annals of Internal Medicine 2009;150:378-95. - PubMed
Kreek 2010
L'Abbé 1987
-
- L'Abbé KA, Detsky AS, O'Rourke K. Meta-analysis in clinical research. Annals of Internal Medicine 1987;107:224-33. - PubMed
Lange 2010
Lunn 2014
Mathew 1999
McNicol 2008
-
- McNicol E. Opioid side effects and their treatment in patients with chronic cancer and noncancer pain. Journal of Pain & Palliative Care Pharmacotherapy 2008;22:270-81. - PubMed
McNicol 2013
McQuay 1998
-
- McQuay H, Moore R. An Evidence-Based Resource for Pain Relief. Oxford: Oxford University Press, 1998. [ISBN: 0-19-263048-2]
McQuay 2007
-
- McQuay HJ, Smith LA, Moore RA. Chronic pain. In: Stevens A, Raftery J, Mant J, Simpson S, editors(s). Health Care Needs Assessment, 3rd Series. Oxford: Radcliffe Publishing, 2007. [ISBN: 978-1-84619-063-6]
Moore 1998
Moore 2008
-
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA, editors(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15-24. [ISBN: 978-0-931092-69-5]
Moore 2009
Moore 2010a
Moore 2010b
Moore 2010c
Moore 2010d
-
- Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta-analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases 2010;69(2):374-9. [DOI: 10.1136/ard.2009.107805] - DOI - PMC - PubMed
Moore 2011a
-
- Moore RA, Straube S, Paine J, Derry S, McQuay HJ. Minimum efficacy criteria for comparisons between treatments using individual patient meta-analysis of acute pain trials: examples of etoricoxib, paracetamol, ibuprofen, and ibuprofen/paracetamol combinations after third molar extraction. Pain 2011;152(5):982-9. - PubMed
Moore 2011b
Moore 2012
Moore 2013a
Moore 2013b
Moore 2014a
Moore 2014b
Moore 2014c
Moore 2015a
Moore 2015b
Moulin 2014
NICE 2013
-
- National Institute for Health and Care Excellence (NICE). Neuropathic pain - pharmacological management: the pharmacological management of neuropathic pain in adults in non-specialist settings, 2013. www.nice.org.uk/guidance/cg173 (accessed 19 October 2014).
Nicholson 2017
Nüesch 2010
O'Brien 2010
PaPaS 2012
-
- Cochrane Pain, Palliative and Supportive Care Group. PaPaS author and referee guidance. papas.cochrane.org/papas-documents (accessed 17 June 2016).
Rappaport 1994
-
- Rappaport ZH, Devor M. Trigeminal neuralgia: the role of self-sustaining discharge in the trigeminal ganglion. Pain 1994;56:127-38. - PubMed
Ray 2015
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ripamonti 1997
Scott 2006
Stannard 2016
Straube 2008
-
- Straube S, Derry S, McQuay HJ, Moore RA. Enriched enrolment: definition and effects of enrichment and dose in trials of pregabalin and gabapentin in neuropathic pain. A systematic review. British Journal of Clinical Pharmacology 2008;66(2):266-75. [DOI: 10.1111/j.1365-2125.2008.03200.] - DOI - PMC - PubMed
Straube 2010
Sultan 2008
Torrance 2006
Trafton 2009
-
- Trafton JA, Ramani A. Methadone: a new old drug with promises and pitfalls. Current Pain and Headache Reports 2009;13:24-30. - PubMed
Treede 2008
van Hecke 2014
van Hoek 2009
von Hehn 2012
Vos 2012
-
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2163-96. [DOI: 10.1016/S0140-6736(12)61729-2] - DOI - PMC - PubMed
Wiffen 2013
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources