KIT Inhibition by Imatinib in Patients with Severe Refractory Asthma
- PMID: 28514613
- PMCID: PMC5568669
- DOI: 10.1056/NEJMoa1613125
KIT Inhibition by Imatinib in Patients with Severe Refractory Asthma
Abstract
Background: Mast cells are present in the airways of patients who have severe asthma despite glucocorticoid treatment; these cells are associated with disease characteristics including poor quality of life and inadequate asthma control. Stem cell factor and its receptor, KIT, are central to mast-cell homeostasis. We conducted a proof-of-principle trial to evaluate the effect of imatinib, a KIT inhibitor, on airway hyperresponsiveness, a physiological marker of severe asthma, as well as on airway mast-cell numbers and activation in patients with severe asthma.
Methods: We conducted a randomized, double-blind, placebo-controlled, 24-week trial of imatinib in patients with poorly controlled severe asthma who had airway hyperresponsiveness despite receiving maximal medical therapy. The primary end point was the change in airway hyperresponsiveness, measured as the concentration of methacholine required to decrease the forced expiratory volume in 1 second by 20% (PC20). Patients also underwent bronchoscopy.
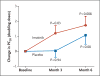
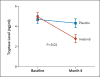
Results: Among the 62 patients who underwent randomization, imatinib treatment reduced airway hyperresponsiveness to a greater extent than did placebo. At 6 months, the methacholine PC20 increased by a mean (±SD) of 1.73±0.60 doubling doses in the imatinib group, as compared with 1.07±0.60 doubling doses in the placebo group (P=0.048). Imatinib also reduced levels of serum tryptase, a marker of mast-cell activation, to a greater extent than did placebo (decrease of 2.02±2.32 vs. 0.56±1.39 ng per milliliter, P=0.02). Airway mast-cell counts declined in both groups. Muscle cramps and hypophosphatemia were more common in the imatinib group than in the placebo group.
Conclusions: In patients with severe asthma, imatinib decreased airway hyperresponsiveness, mast-cell counts, and tryptase release. These results suggest that KIT-dependent processes and mast cells contribute to the pathobiologic basis of severe asthma. (Funded by the National Institutes of Health and others; ClinicalTrials.gov number, NCT01097694 .).
Figures
Comment in
-
Mast Cells and KIT as Potential Therapeutic Targets in Severe Asthma.N Engl J Med. 2017 May 18;376(20):1983-1984. doi: 10.1056/NEJMe1702653. N Engl J Med. 2017. PMID: 28514622 No abstract available.
-
Novel Treatments for Airway Disease.N Engl J Med. 2017 Aug 10;377(6):596. doi: 10.1056/NEJMc1708004. N Engl J Med. 2017. PMID: 28792870 No abstract available.
-
Novel Treatments for Airway Disease.N Engl J Med. 2017 Aug 10;377(6):595-6. doi: 10.1056/NEJMc1708004. N Engl J Med. 2017. PMID: 28812851 No abstract available.
References
-
- Wenzel S. Severe asthma in adults. Am J Respir Crit Care Med. 2005;172:149–60. - PubMed
-
- Chanez P, Wenzel SE, Anderson GP, et al. Severe asthma in adults: what are the important questions? J Allergy Clin Immunol. 2007;119:1337–48. - PubMed
-
- Busse WW, Banks-Schlegel S, Wenzel SE. Pathophysiology of severe asthma. J Allergy Clin Immunol. 2000;106:1033–42. - PubMed
-
- Limb SL, Brown KC, Wood RA, et al. Irreversible lung function deficits in young adults with a history of childhood asthma. J Allergy Clin Immunol. 2005;116:1213–9. - PubMed
-
- Porsbjerg C, Rasmussen L, Nolte H, Backer V. Association of airway hyperresponsiveness with reduced quality of life in patients with moderate to severe asthma. Ann Allergy Asthma Immunol. 2007;98:44–50. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
