MenTORing Immunity: mTOR Signaling in the Development and Function of Tissue-Resident Immune Cells
- PMID: 28514674
- PMCID: PMC5695239
- DOI: 10.1016/j.immuni.2017.04.028
MenTORing Immunity: mTOR Signaling in the Development and Function of Tissue-Resident Immune Cells
Abstract
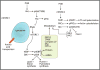
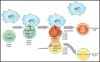
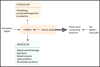
Tissue-resident immune cells must balance survival in peripheral tissues with the capacity to respond rapidly upon infection or tissue damage, and in turn couple these responses with intrinsic metabolic control and conditions in the tissue microenvironment. The serine/threonine kinase mammalian/mechanistic target of rapamycin (mTOR) is a central integrator of extracellular and intracellular growth signals and cellular metabolism and plays important roles in both innate and adaptive immune responses. This review discusses the function of mTOR signaling in the differentiation and function of tissue-resident immune cells, with focus on the role of mTOR as a metabolic sensor and its impact on metabolic regulation in innate and adaptive immune cells. We also discuss the impact of metabolic constraints in tissues on immune homeostasis and disease, and how manipulating mTOR activity with drugs such as rapamycin can modulate immunity in these contexts.
Copyright © 2017. Published by Elsevier Inc.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

