Glycine Substitution at Helix-to-Coil Transitions Facilitates the Structural Determination of a Stabilized Subtype C HIV Envelope Glycoprotein
- PMID: 28514686
- PMCID: PMC5439057
- DOI: 10.1016/j.immuni.2017.04.014
Glycine Substitution at Helix-to-Coil Transitions Facilitates the Structural Determination of a Stabilized Subtype C HIV Envelope Glycoprotein
Abstract
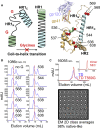
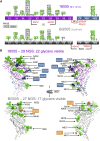
Advances in HIV-1 envelope glycoprotein (Env) design generate native-like trimers and high-resolution clade A, B, and G structures and elicit neutralizing antibodies. However, a high-resolution clade C structure is critical, as this subtype accounts for the majority of HIV infections worldwide, but well-ordered clade C Env trimers are more challenging to produce due to their instability. Based on targeted glycine substitutions in the Env fusion machinery, we defined a general approach that disfavors helical transitions leading to post-fusion conformations, thereby favoring the pre-fusion state. We generated a stabilized, soluble clade C Env (16055 NFL) and determined its crystal structure at 3.9 Å. Its overall conformation is similar to SOSIP.664 and native Env trimers but includes a covalent linker between gp120 and gp41, an engineered 201-433 disulfide bond, and density corresponding to 22 N-glycans. Env-structure-guided design strategies resulted in multiple homogeneous cross-clade immunogens with the potential to advance HIV vaccine development.
Keywords: Antibody; Envelope glycoprotein; Glycan shield; HIV; Immunogen; Trimer; Vaccine; bNAb.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Berger E.A., Lifson J.D., Eiden L.E. Stimulation of glycoprotein gp120 dissociation from the envelope glycoprotein complex of human immunodeficiency virus type 1 by soluble CD4 and CD4 peptide derivatives: implications for the role of the complementarity-determining region 3-like region in membrane fusion. Proc. Natl. Acad. Sci. USA. 1991;88:8082–8086. - PMC - PubMed
-
- Binley J.M., Sanders R.W., Clas B., Schuelke N., Master A., Guo Y., Kajumo F., Anselma D.J., Maddon P.J., Olson W.C., Moore J.P. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J. Virol. 2000;74:627–643. - PMC - PubMed
-
- Colman P.M., Lawrence M.C. The structural biology of type I viral membrane fusion. Nat. Rev. Mol. Cell Biol. 2003;4:309–319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

