Flexible ammonia handling strategies using both cutaneous and branchial epithelia in the highly ammonia-tolerant Pacific hagfish
- PMID: 28515081
- PMCID: PMC5582951
- DOI: 10.1152/ajpregu.00351.2016
Flexible ammonia handling strategies using both cutaneous and branchial epithelia in the highly ammonia-tolerant Pacific hagfish
Abstract
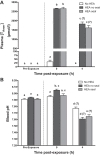
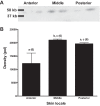
Hagfish consume carrion, potentially exposing them to hypoxia, hypercapnia, and high environmental ammonia (HEA). We investigated branchial and cutaneous ammonia handling strategies by which Pacific hagfish (Eptatretus stoutii) tolerate and recover from high ammonia loading. Hagfish were exposed to HEA (20 mmol/l) for 48 h to elevate plasma total ammonia (TAmm) levels before placement into divided chambers for a 4-h recovery period in ammonia-free seawater where ammonia excretion (JAmm) was measured independently in the anterior and posterior compartments. Localized HEA exposures were also conducted by subjecting hagfish to HEA in either the anterior or posterior compartments. During recovery, HEA-exposed animals increased JAmm in both compartments, with the posterior compartment comprising ~20% of the total JAmm compared with ~11% in non-HEA-exposed fish. Plasma TAmm increased substantially when whole hagfish and the posterior regions were exposed to HEA. Alternatively, plasma TAmm did not elevate after anterior localized HEA exposure. JAmm was concentration dependent (0.05-5 mmol/l) across excised skin patches at up to eightfold greater rates than in skin sections that were excised from HEA-exposed hagfish. Skin excised from more posterior regions displayed greater JAmm than those from more anterior regions. Immunohistochemistry with hagfish-specific anti-rhesus glycoprotein type c (α-hRhcg; ammonia transporter) antibody was characterized by staining on the basal aspect of hagfish epidermis while Western blotting demonstrated greater expression of Rhcg in more posterior skin sections. We conclude that cutaneous Rhcg proteins are involved in cutaneous ammonia excretion by Pacific hagfish and that this mechanism could be particularly important during feeding.
Keywords: agnatha; cyclostome; nitrogen; rhesus glycoprotein; skin.
Copyright © 2017 the American Physiological Society.
Figures






Comment in
-
Heads you gain, tails you lose.Am J Physiol Regul Integr Comp Physiol. 2017 Aug 1;313(2):R65-R66. doi: 10.1152/ajpregu.00208.2017. Epub 2017 Jun 7. Am J Physiol Regul Integr Comp Physiol. 2017. PMID: 28592460 Free PMC article. No abstract available.
References
-
- Alt JM, Stolte H, Eisenbach GM, Walvig F. Renal electrolyte and fluid excretion in the Atlantic hagfish Myxine glutinosa. J Exp Biol 91: 323–330, 1981.
-
- Boutilier RG, Heming TA, Iwama GK. Appendix: physicochemical parameters for use in fish respiratory physiology In: Gills–Anatomy, Gas Transfer, and Acid-Base Regulation, edited by Hoar WS, Randall D. Orlando, FL: Elsevier, 1984, p. 403–430. doi: 10.1016/S1546-5098(08)60323-4. - DOI
-
- Cameron JN, Heisler N. Studies of ammonia in the Rainbow trout: Physico-chemical parameters, acid-base behaviour and respiratory clearance. J Exp Biol 105: 107–125, 1983.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

