Circulating soluble P-selectin must dimerize to promote inflammation and coagulation in mice
- PMID: 28515093
- PMCID: PMC5510792
- DOI: 10.1182/blood-2017-02-770479
Circulating soluble P-selectin must dimerize to promote inflammation and coagulation in mice
Abstract
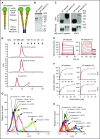
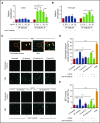
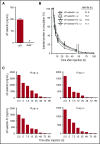
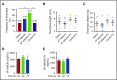
Leukocyte adhesion to P-selectin on activated platelets and endothelial cells induces shedding of the P-selectin ectodomain into the circulation. Plasma soluble P-selectin (sP-selectin) is elevated threefold to fourfold in patients with cardiovascular disease. Circulating sP-selectin is thought to trigger signaling in leukocytes that directly contributes to inflammation and thrombosis. However, sP-selectin likely circulates as a monomer, and in vitro studies suggest that sP-selectin must dimerize to induce signaling in leukocytes. To address this discrepancy, we expressed the entire ectodomain of mouse P-selectin as a monomer (sP-selectin) or as a disulfide-linked dimer fused to the Fc portion of mouse immunoglobulin G (sP-selectin-Fc). Dimeric sP-selectin-Fc, but not monomeric sP-selectin, triggered integrin-dependent adhesion of mouse leukocytes in vitro. Antibody-induced oligomerization of sP-selectin or sP-selectin-Fc was required to trigger formation of neutrophil extracellular traps. Injecting sP-selectin-Fc, but not sP-selectin, into mice augmented integrin-dependent adhesion of neutrophils in venules, generated tissue factor-bearing microparticles, shortened plasma-clotting times, and increased thrombus frequency in the inferior vena cava. Furthermore, transgenic mice that overexpressed monomeric sP-selectin did not exhibit increased inflammation or thrombosis. We conclude that elevated plasma sP-selectin is a consequence rather than a cause of cardiovascular disease.
© 2017 by The American Society of Hematology.
Figures


Comment in
-
Soluble P-selectin is the smoke, not the fire.Blood. 2017 Jul 13;130(2):101-102. doi: 10.1182/blood-2017-05-786319. Blood. 2017. PMID: 28705855 No abstract available.
Similar articles
-
Endothelial PPAR-γ protects against vascular thrombosis by downregulating P-selectin expression.Arterioscler Thromb Vasc Biol. 2015 Apr;35(4):838-44. doi: 10.1161/ATVBAHA.115.305378. Epub 2015 Feb 12. Arterioscler Thromb Vasc Biol. 2015. PMID: 25675995 Free PMC article.
-
P-selectin promotes neutrophil extracellular trap formation in mice.Blood. 2015 Jul 9;126(2):242-6. doi: 10.1182/blood-2015-01-624023. Epub 2015 May 15. Blood. 2015. PMID: 25979951 Free PMC article.
-
Distinct contributions of complement factors to platelet activation and fibrin formation in venous thrombus development.Blood. 2017 Apr 20;129(16):2291-2302. doi: 10.1182/blood-2016-11-749879. Epub 2017 Feb 21. Blood. 2017. PMID: 28223279 Free PMC article.
-
Platelet-leukocyte aggregates and derived microparticles in inflammation, vascular remodelling and thrombosis.Front Biosci. 2006 Jan 1;11:830-7. doi: 10.2741/1840. Front Biosci. 2006. PMID: 16146774 Review.
-
P-selectin mediates adhesion of leukocytes, platelets, and cancer cells in inflammation, thrombosis, and cancer growth and metastasis.Arch Immunol Ther Exp (Warsz). 2006 Mar-Apr;54(2):75-84. doi: 10.1007/s00005-006-0010-6. Epub 2006 Mar 24. Arch Immunol Ther Exp (Warsz). 2006. PMID: 16648968 Review.
Cited by
-
PAD4 and Its Inhibitors in Cancer Progression and Prognosis.Pharmaceutics. 2022 Nov 8;14(11):2414. doi: 10.3390/pharmaceutics14112414. Pharmaceutics. 2022. PMID: 36365233 Free PMC article. Review.
-
Covid-19: The Rollercoaster of Fibrin(Ogen), D-Dimer, Von Willebrand Factor, P-Selectin and Their Interactions with Endothelial Cells, Platelets and Erythrocytes.Int J Mol Sci. 2020 Jul 21;21(14):5168. doi: 10.3390/ijms21145168. Int J Mol Sci. 2020. PMID: 32708334 Free PMC article. Review.
-
P-Selectin Level at First and Third Day After Portal Hypertensive Splenectomy for Early Prediction of Portal Vein Thrombosis in Patients With Cirrhosis.Clin Appl Thromb Hemost. 2018 Dec;24(9_suppl):76S-83S. doi: 10.1177/1076029618788180. Epub 2018 Jul 22. Clin Appl Thromb Hemost. 2018. PMID: 30033741 Free PMC article. Clinical Trial.
-
Chronic Immune Platelet Activation Is Followed by Platelet Refractoriness and Impaired Contractility.Int J Mol Sci. 2022 Jun 30;23(13):7336. doi: 10.3390/ijms23137336. Int J Mol Sci. 2022. PMID: 35806341 Free PMC article.
-
Exploration on the effect of anserine on the alleviation of DVT and its molecular mechanism.Front Pharmacol. 2024 May 23;15:1402758. doi: 10.3389/fphar.2024.1402758. eCollection 2024. Front Pharmacol. 2024. PMID: 38846090 Free PMC article.
References
-
- Ushiyama S, Laue TM, Moore KL, Erickson HP, McEver RP. Structural and functional characterization of monomeric soluble P-selectin and comparison with membrane P-selectin. J Biol Chem. 1993;268(20):15229-15237. - PubMed
-
- Barkalow FJ, Barkalow KL, Mayadas TN. Dimerization of P-selectin in platelets and endothelial cells. Blood. 2000;96(9):3070-3077. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

