NEMO, a Transcriptional Target of Estrogen and Progesterone, Is Linked to Tumor Suppressor PML in Breast Cancer
- PMID: 28515148
- PMCID: PMC8236416
- DOI: 10.1158/0008-5472.CAN-16-2794
NEMO, a Transcriptional Target of Estrogen and Progesterone, Is Linked to Tumor Suppressor PML in Breast Cancer
Abstract
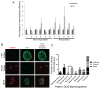
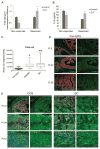
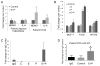
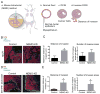
The beneficial versus detrimental roles of estrogen plus progesterone (E+P) in breast cancer remains controversial. Here we report a beneficial mechanism of E+P treatment in breast cancer cells driven by transcriptional upregulation of the NFκB modulator NEMO, which in turn promotes expression of the tumor suppressor protein promyelocytic leukemia (PML). E+P treatment of patient-derived epithelial cells derived from ductal carcinoma in situ (DCIS) increased secretion of the proinflammatory cytokine IL6. Mechanistic investigations indicated that IL6 upregulation occurred as a result of transcriptional upregulation of NEMO, the gene that harbored estrogen receptor (ER) binding sites within its promoter. Accordingly, E+P treatment of breast cancer cells increased ER binding to the NEMO promoter, thereby increasing NEMO expression, NFκB activation, and IL6 secretion. In two mouse xenograft models of DCIS, we found that RNAi-mediated silencing of NEMO increased tumor invasion and progression. This seemingly paradoxical result was linked to NEMO-mediated regulation of NFκB and IL6 secretion, increased phosphorylation of STAT3 on Ser727, and increased expression of PML, a STAT3 transcriptional target. In identifying NEMO as a pivotal transcriptional target of E+P signaling in breast cancer cells, our work offers a mechanistic explanation for the paradoxical antitumorigenic roles of E+P in breast cancer by showing how it upregulates the tumor suppressor protein PML. Cancer Res; 77(14); 3802-13. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Figures

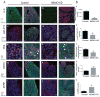
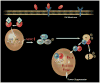
Similar articles
-
Interleukin 6 signaling regulates promyelocytic leukemia protein gene expression in human normal and cancer cells.J Biol Chem. 2012 Aug 3;287(32):26702-14. doi: 10.1074/jbc.M111.316869. Epub 2012 Jun 18. J Biol Chem. 2012. PMID: 22711534 Free PMC article.
-
The PML1-WDR5 axis regulates H3K4me3 marks and promotes stemness of estrogen receptor-positive breast cancer.Cell Death Differ. 2024 Jun;31(6):768-778. doi: 10.1038/s41418-024-01294-6. Epub 2024 Apr 16. Cell Death Differ. 2024. PMID: 38627584 Free PMC article.
-
Estrogen receptor alpha transcriptionally activates casein kinase 2 alpha: A pivotal regulator of promyelocytic leukaemia protein (PML) and AKT in oncogenesis.Cell Signal. 2016 Jun;28(6):675-87. doi: 10.1016/j.cellsig.2016.03.007. Epub 2016 Mar 21. Cell Signal. 2016. PMID: 27012497
-
The Emerging Roles of Steroid Hormone Receptors in Ductal Carcinoma in Situ (DCIS) of the Breast.J Mammary Gland Biol Neoplasia. 2018 Dec;23(4):237-248. doi: 10.1007/s10911-018-9416-0. Epub 2018 Oct 18. J Mammary Gland Biol Neoplasia. 2018. PMID: 30338425 Free PMC article. Review.
-
Mechanisms of hormonal prevention of breast cancer.Ann N Y Acad Sci. 2001 Dec;952:23-35. doi: 10.1111/j.1749-6632.2001.tb02725.x. Ann N Y Acad Sci. 2001. PMID: 11795441 Review.
Cited by
-
SUMOylation Wrestles With the Occurrence and Development of Breast Cancer.Front Oncol. 2021 Apr 21;11:659661. doi: 10.3389/fonc.2021.659661. eCollection 2021. Front Oncol. 2021. PMID: 33968766 Free PMC article. Review.
-
Placental Expression of NEMO Protein in Normal Pregnancy and Preeclampsia.Dis Markers. 2019 Jan 2;2019:8418379. doi: 10.1155/2019/8418379. eCollection 2019. Dis Markers. 2019. PMID: 30723530 Free PMC article.
-
EDA-ID: a Severe Clinical Presentation Associated with a New IKBKG Mutation.J Clin Immunol. 2021 Jul;41(5):1099-1102. doi: 10.1007/s10875-021-00992-x. Epub 2021 Feb 17. J Clin Immunol. 2021. PMID: 33598805 Free PMC article. No abstract available.
-
Molecular signatures of in situ to invasive progression for basal-like breast cancers: An integrated mouse model and human DCIS study.NPJ Breast Cancer. 2022 Jul 18;8(1):83. doi: 10.1038/s41523-022-00450-w. NPJ Breast Cancer. 2022. PMID: 35851387 Free PMC article.
-
Progesterone and Breast Cancer: an NCI Workshop Report.Horm Cancer. 2020 Feb;11(1):1-12. doi: 10.1007/s12672-020-00379-1. Horm Cancer. 2020. PMID: 32016820 Free PMC article. No abstract available.
References
-
- Allegra CJ, Aberle DR, Ganschow P, Hahn SM, Lee CN, Millon-Underwood S, et al. National Institutes of Health State-of-the-Science Conference statement: Diagnosis and Management of Ductal Carcinoma In Situ September 22–24, 2009. J Natl Cancer Inst. 2010;102(3):161–9. - PubMed
-
- Virnig BA, Tuttle TM, Shamliyan T, Kane RL. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst. 2010;102(3):170–8. - PubMed
-
- Leonard GD, Swain SM. Ductal carcinoma in situ, complexities and challenges. J Natl Cancer Inst. 2004;96(12):906–20. - PubMed
-
- Ewertz M, Duffy SW, Adami HO, Kvale G, Lund E, Meirik O, et al. Age at first birth, parity and risk of breast cancer: a meta-analysis of 8 studies from the Nordic countries. Int J Cancer. 1990;46(4):597–603. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

