Regulation of WNT Signaling at the Neuromuscular Junction by the Immunoglobulin Superfamily Protein RIG-3 in Caenorhabditis elegans
- PMID: 28515212
- PMCID: PMC5500148
- DOI: 10.1534/genetics.116.195297
Regulation of WNT Signaling at the Neuromuscular Junction by the Immunoglobulin Superfamily Protein RIG-3 in Caenorhabditis elegans
Abstract
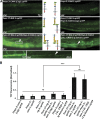
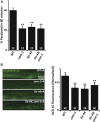
Perturbations in synaptic function could affect the normal behavior of an animal, making it important to understand the regulatory mechanisms of synaptic signaling. Previous work has shown that in Caenorhabditis elegans an immunoglobulin superfamily protein, RIG-3, functions in presynaptic neurons to maintain normal acetylcholine receptor levels at the neuromuscular junction (NMJ). In this study, we elucidate the molecular and functional mechanism of RIG-3. We demonstrate by genetic and BiFC (Bi-molecular Fluorescence Complementation) assays that presynaptic RIG-3 functions by directly interacting with the immunoglobulin domain of the nonconventional Wnt receptor, ROR receptor tyrosine kinase (RTK), CAM-1, which functions in postsynaptic body-wall muscles. This interaction in turn inhibits Wnt/LIN-44 signaling through the ROR/CAM-1 receptor, and allows for maintenance of normal acetylcholine receptor, AChR/ACR-16, levels at the neuromuscular synapse. Further, this work reveals that RIG-3 and ROR/CAM-1 function through the β-catenin/HMP-2 at the NMJ. Taken together, our results demonstrate that RIG-3 functions as an inhibitory molecule of the Wnt/LIN-44 signaling pathway through the RTK, CAM-1.
Keywords: C. elegans; RIG-3; Wnt; immunoglobulin domain; neuromuscular junction.
Copyright © 2017 by the Genetics Society of America.
Figures




References
-
- Bei Y., Hogan J., Berkowitz L. A., Soto M., Rocheleau C. E., et al. , 2002. SRC-1 and Wnt signaling act together to specify endoderm and to control cleavage orientation in early C. elegans embryos. Dev. Cell 3: 113–125. - PubMed
-
- Bielen H., Houart C., 2014. The Wnt cries many: Wnt regulation of neurogenesis through tissue patterning, proliferation, and asymmetric cell division. Dev. Neurobiol. 74: 772–780. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

