Assessment of the Cavidi ExaVir Load Assay for Monitoring Plasma Viral Load in HIV-2-Infected Patients
- PMID: 28515216
- PMCID: PMC5527414
- DOI: 10.1128/JCM.00235-17
Assessment of the Cavidi ExaVir Load Assay for Monitoring Plasma Viral Load in HIV-2-Infected Patients
Abstract
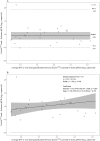
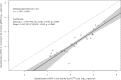
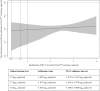
HIV plasma viral load is an established marker of disease progression and of response to antiretroviral therapy, but currently there is no commercial assay validated for the quantification of viral load in HIV-2-infected individuals. We sought to make the first clinical evaluation of Cavidi ExaVir Load (version 3) in HIV-2-infected patients. Samples were collected from a total of 102 individuals living in Cape Verde, and the HIV-2 viral load was quantified by both ExaVir Load and a reference in-house real-time quantitative PCR (qPCR) used in Portugal in 91 samples. The associations between viral load and clinical prognostic variables (CD4+ T cell counts and antiretroviral therapy status) were similar for measurements obtained using ExaVir Load and qPCR. There was no difference between the two methods in the capacity to discriminate between nonquantifiable and quantifiable HIV-2 in the plasma. In samples with an HIV-2 viral load quantifiable by both methods (n = 27), the measurements were highly correlated (Pearson r = 0.908), but the ExaVir Load values were systematically higher relative to those determined by qPCR (median difference, 0.942 log10 copies/ml). A regression model was derived that enables the conversion of ExaVir Load results to those that would have been obtained by the reference qPCR. In conclusion, ExaVir Load version 3 is a reliable commercial assay to measure viral load in HIV-2-infected patients and therefore a valuable alternative to the in-house assays in current use.
Keywords: Cape Verde; Cavidi ExaVir load; HIV-2; resource-limited settings; viral load assay.
Copyright © 2017 American Society for Microbiology.
Figures
References
-
- DHHS-OARAC. 2016. Panel on antiretroviral guidelines for adults and adolescents: guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. DHHS-OARAC, Bethesda, MD: http://www.aidsinfo.nih.gov/guidelines.
-
- Damond F, Benard A, Ruelle J, Alabi A, Kupfer B, Gomes P, Rodes B, Albert J, Boni J, Garson J, Ferns B, Matheron S, Chene G, Brun-Vezinet F. 2008. Quality control assessment of human immunodeficiency virus type 2 (HIV-2) viral load quantification assays: results from an international collaboration on HIV-2 infection in 2006. J Clin Microbiol 46:2088–2091. doi:10.1128/JCM.00126-08. - DOI - PMC - PubMed
-
- Damond F, Benard A, Balotta C, Boni J, Cotten M, Duque V, Ferns B, Garson J, Gomes P, Gonçalves F, Gottlieb G, Kupfer B, Ruelle J, Rodes B, Soriano V, Wainberg M, Taieb A, Matheron S, Chene G, Brun-Vezinet F. 2011. An international collaboration to standardize HIV-2 viral load assays: results from the 2009 ACHI EV 2E quality control study. J Clin Microbiol 49:3491–3497. doi:10.1128/JCM.02389-10. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

