A Novel A(H7N2) Influenza Virus Isolated from a Veterinarian Caring for Cats in a New York City Animal Shelter Causes Mild Disease and Transmits Poorly in the Ferret Model
- PMID: 28515300
- PMCID: PMC5512233
- DOI: 10.1128/JVI.00672-17
A Novel A(H7N2) Influenza Virus Isolated from a Veterinarian Caring for Cats in a New York City Animal Shelter Causes Mild Disease and Transmits Poorly in the Ferret Model
Abstract
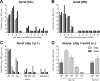
In December 2016, a low-pathogenic avian influenza (LPAI) A(H7N2) virus was identified to be the causative source of an outbreak in a cat shelter in New York City, which subsequently spread to multiple shelters in the states of New York and Pennsylvania. One person with occupational exposure to infected cats became infected with the virus, representing the first LPAI H7N2 virus infection in a human in North America since 2003. Considering the close contact that frequently occurs between companion animals and humans, it was critical to assess the relative risk of this novel virus to public health. The virus isolated from the human case, A/New York/108/2016 (NY/108), caused mild and transient illness in ferrets and mice but did not transmit to naive cohoused ferrets following traditional or aerosol-based inoculation methods. The environmental persistence of NY/108 virus was generally comparable to that of other LPAI H7N2 viruses. However, NY/108 virus replicated in human bronchial epithelial cells with an increased efficiency compared with that of previously isolated H7N2 viruses. Furthermore, the novel H7N2 virus was found to utilize a relatively lower pH for hemagglutinin activation, similar to human influenza viruses. Our data suggest that the LPAI H7N2 virus requires further adaptation before representing a substantial threat to public health. However, the reemergence of an LPAI H7N2 virus in the northeastern United States underscores the need for continuous surveillance of emerging zoonotic influenza viruses inclusive of mammalian species, such as domestic felines, that are not commonly considered intermediate hosts for avian influenza viruses.IMPORTANCE Avian influenza viruses are capable of crossing the species barrier to infect mammals, an event of public health concern due to the potential acquisition of a pandemic phenotype. In December 2016, an H7N2 virus caused an outbreak in cats in multiple animal shelters in New York State. This was the first detection of this virus in the northeastern United States in over a decade and the first documented infection of a felid with an H7N2 virus. A veterinarian became infected following occupational exposure to H7N2 virus-infected cats, necessitating the evaluation of this virus for its capacity to cause disease in mammals. While the H7N2 virus was associated with mild illness in mice and ferrets and did not spread well between ferrets, it nonetheless possessed several markers of virulence for mammals. These data highlight the promiscuity of influenza viruses and the need for diligent surveillance across multiple species to quickly identify an emerging strain with pandemic potential.
Keywords: H7N2; cats; ferret; influenza; low-pathogenic avian influenza virus; pathogenesis; transmission.
Copyright © 2017 American Society for Microbiology.
Figures
Similar articles
-
Influenza Virus Infections in Cats.Viruses. 2021 Jul 23;13(8):1435. doi: 10.3390/v13081435. Viruses. 2021. PMID: 34452300 Free PMC article. Review.
-
Characterization of a Feline Influenza A(H7N2) Virus.Emerg Infect Dis. 2018 Jan;24(1):75-86. doi: 10.3201/eid2401.171240. Emerg Infect Dis. 2018. PMID: 29260686 Free PMC article.
-
Detection of an avian lineage influenza A(H7N2) virus in air and surface samples at a New York City feline quarantine facility.Influenza Other Respir Viruses. 2018 Sep;12(5):613-622. doi: 10.1111/irv.12572. Epub 2018 Jun 30. Influenza Other Respir Viruses. 2018. PMID: 29768714 Free PMC article.
-
Outbreak of Influenza A(H7N2) Among Cats in an Animal Shelter With Cat-to-Human Transmission-New York City, 2016.Clin Infect Dis. 2017 Nov 13;65(11):1927-1929. doi: 10.1093/cid/cix668. Clin Infect Dis. 2017. PMID: 29020187
-
Canine and Feline Influenza.Cold Spring Harb Perspect Med. 2021 Jan 4;11(1):a038562. doi: 10.1101/cshperspect.a038562. Cold Spring Harb Perspect Med. 2021. PMID: 31871238 Free PMC article. Review.
Cited by
-
Influenza Virus Infections in Cats.Viruses. 2021 Jul 23;13(8):1435. doi: 10.3390/v13081435. Viruses. 2021. PMID: 34452300 Free PMC article. Review.
-
Risk Assessment of Fifth-Wave H7N9 Influenza A Viruses in Mammalian Models.J Virol. 2018 Dec 10;93(1):e01740-18. doi: 10.1128/JVI.01740-18. Print 2019 Jan 1. J Virol. 2018. PMID: 30305359 Free PMC article.
-
Emergence and Characterization of a Novel Reassortant Canine Influenza Virus Isolated from Cats.Pathogens. 2021 Oct 14;10(10):1320. doi: 10.3390/pathogens10101320. Pathogens. 2021. PMID: 34684269 Free PMC article.
-
Evidence of Influenza A in Wild Norway Rats (Rattus norvegicus) in Boston, Massachusetts.Front Ecol Evol. 2019 Mar;7:36. doi: 10.3389/fevo.2019.00036. Epub 2019 Mar 14. Front Ecol Evol. 2019. PMID: 34660611 Free PMC article.
-
Genetic characterization of an H3N2 canine influenza virus strain in China in 2023-acquisition of novel human-like amino acid substitutions.Front Vet Sci. 2025 Mar 3;12:1552115. doi: 10.3389/fvets.2025.1552115. eCollection 2025. Front Vet Sci. 2025. PMID: 40098892 Free PMC article.
References
-
- Senne D. 2007. National Veterinary Services Laboratory avian influenza and Newcastle disease activities FY 2007. National Veterinary Services Laboratory, Ames, IA.
-
- CDC. 2004. Update: influenza activity—United States, 2003–04 season. MMWR Morb Mortal Wkly Rep 53:284–287. - PubMed
-
- Ostrowsky B, Huang A, Terry W, Anton D, Brunagel B, Traynor L, Abid S, Johnson G, Kacica M, Katz J, Edwards L, Lindstrom S, Klimov A, Uyeki TM. 2012. Low pathogenic avian influenza A (H7N2) virus infection in immunocompromised adult, New York, USA, 2003. Emerg Infect Dis 18:1128–1131. doi:10.3201/eid1807.111913. - DOI - PMC - PubMed
-
- Anonymous. 2007. Avian influenza A/(H7N2) outbreak in the United Kingdom. Euro Surveill 12(22):pii=3206 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=3206. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

