The C-terminal peptide of Aquifex aeolicus riboflavin synthase directs encapsulation of native and foreign guests by a cage-forming lumazine synthase
- PMID: 28515315
- PMCID: PMC5481547
- DOI: 10.1074/jbc.C117.790311
The C-terminal peptide of Aquifex aeolicus riboflavin synthase directs encapsulation of native and foreign guests by a cage-forming lumazine synthase
Abstract
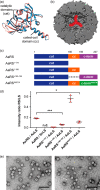
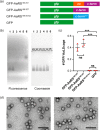
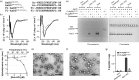
Encapsulation of specific enzymes in self-assembling protein cages is a hallmark of bacterial compartments that function as counterparts to eukaryotic organelles. The cage-forming enzyme lumazine synthase (LS) from Bacillus subtilis (BsLS), for example, encapsulates riboflavin synthase (BsRS), enabling channeling of lumazine from the site of its generation to the site of its conversion to vitamin B2 Elucidating the molecular mechanisms underlying the assembly of these supramolecular complexes could help inform new approaches for metabolic engineering, nanotechnology, and drug delivery. To that end, we investigated a thermostable LS from Aquifex aeolicus (AaLS) and found that it also forms cage complexes with the cognate riboflavin synthase (AaRS) when both proteins are co-produced in the cytosol of Escherichia coli A 12-amino acid-long peptide at the C terminus of AaRS serves as a specific localization sequence responsible for targeting the guest to the protein compartment. Sequence comparisons suggested that analogous peptide segments likely direct RS complexation by LS cages in other bacterial species. Covalent fusion of this peptide tag to heterologous guest molecules led to their internalization into AaLS assemblies both in vivo and in vitro, providing a firm foundation for creating tailored biomimetic nanocompartments for medical and biotechnological applications.
Keywords: bacterial compartment; biotechnology; cell compartmentalization; localization sequence; lumazine/riboflavin synthase; protein cage; protein self-assembly; protein-protein interaction; synthetic biology.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
References
-
- Bobik T. A. (2006) Polyhedral organelles compartmenting bacterial metabolic processes. Appl. Microbiol. Biotechnol. 70, 517–525 - PubMed
-
- Yeates T. O., Kerfeld C. A., Heinhorst S., Cannon G. C., and Shively J. M. (2008) Protein-based organelles in bacteria: carboxysomes and related microcompartments. Nat. Rev. Microbiol. 6, 681–691 - PubMed
-
- Kerfeld C. A., Heinhorst S., and Cannon G. C. (2010) Bacterial microcompartments. Annu. Rev. Microbiol. 64, 391–408 - PubMed
-
- Sutter M., Boehringer D., Gutmann S., Günther S., Prangishvili D., Loessner M. J., Stetter K. O., Weber-Ban E., and Ban N. (2008) Structural basis of enzyme encapsulation into a bacterial nanocompartment. Nat. Struct. Mol. Biol. 15, 939–947 - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

