Molecular mechanism of activation of class IA phosphoinositide 3-kinases (PI3Ks) by membrane-localized HRas
- PMID: 28515318
- PMCID: PMC5519374
- DOI: 10.1074/jbc.M117.789263
Molecular mechanism of activation of class IA phosphoinositide 3-kinases (PI3Ks) by membrane-localized HRas
Abstract
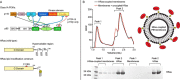
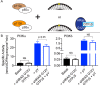
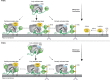
Class IA PI3Ks are involved in the generation of the key lipid signaling molecule phosphatidylinositol 3,4,5-trisphosphate (PIP3), and inappropriate activation of this pathway is implicated in a multitude of human diseases, including cancer, inflammation, and primary immunodeficiencies. Class IA PI3Ks are activated downstream of the Ras superfamily of GTPases, and Ras-PI3K interaction plays a key role in promoting tumor formation and maintenance in Ras-driven tumors. Investigating the detailed molecular events in the Ras-PI3K interaction has been challenging because it occurs on a membrane surface. Here, using maleimide-functionalized lipid vesicles, we successfully generated membrane-resident HRas and evaluated its effect on PI3K signaling in lipid kinase assays and through analysis with hydrogen-deuterium exchange MS. We screened all class IA PI3K isoforms and found that HRas activates both p110α and p110δ isoforms but does not activate p110β. The p110α and p110δ activation by Ras was synergistic with activation by a soluble phosphopeptide derived from receptor tyrosine kinases. Hydrogen-deuterium exchange MS revealed that membrane-resident HRas, but not soluble HRas, enhances conformational changes associated with membrane binding by increasing membrane recruitment of both p110α and p110δ. Together, these results afford detailed molecular insight into the Ras-PI3K signaling complex, provide a framework for screening Ras inhibitors, and shed light on the isoform specificity of Ras-PI3K interactions in a native membrane context.
Keywords: Ras protein; hydrogen exchange mass spectrometry; lipid signaling; lipid–protein interaction; phosphatidylinositide 3-kinase (PI 3-kinase); phosphoinositide.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
-
- Vanhaesebroeck B., Stephens L., and Hawkins P. (2012) PI3K signalling: the path to discovery and understanding. Nat. Rev. Mol. Cell Biol. 13, 195–203 - PubMed
-
- Burke J. E., and Williams R. L. (2015) Synergy in activating class I PI3Ks. Trends Biochem. Sci. 40, 88–100 - PubMed
-
- Rodriguez-Viciana P., Warne P. H., Dhand R., Vanhaesebroeck B., Gout I., Fry M. J., Waterfield M. D., and Downward J. (1994) Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature 370, 527–532 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

