Flavin-containing monooxygenases in aging and disease: Emerging roles for ancient enzymes
- PMID: 28515321
- PMCID: PMC5500783
- DOI: 10.1074/jbc.R117.779678
Flavin-containing monooxygenases in aging and disease: Emerging roles for ancient enzymes
Abstract
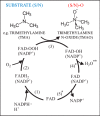
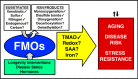
Flavin-containing monooxygenases (FMOs) are primarily studied as xenobiotic metabolizing enzymes with a prominent role in drug metabolism. In contrast, endogenous functions and substrates of FMOs are less well understood. A growing body of recent evidence, however, implicates FMOs in aging, several diseases, and metabolic pathways. The evidence suggests an important role for these well-conserved proteins in multiple processes and raises questions about the endogenous substrate(s) and regulation of FMOs. Here, we present an overview of evidence for FMOs' involvement in aging and disease, discussing the biological context and arguing for increased investigation into the function of these enzymes.
Keywords: aging; atherosclerosis; flavin-containing monooxygenase (FMO); flavoprotein; iron; metabolism; neurodegenerative disease; oxidation–reduction (redox); sulfur; trimethylamine–N-oxide (TMAO); xenobiotic; xenobiotic metabolism.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
References
-
- Huijbers M. M. E., Montersino S., Westphal A. H., Tischler D., and Van Berkel W. J. H. (2014) Flavin dependent monooxygenases. Arch. Biochem. Biophys. 544, 2–17 - PubMed
-
- Ziegler D. M., Duffel M. W., and Poulsen L. L. (1979) Studies on the nature and regulation of the cellular thiol:disulphide potential. Ciba Found. Symp. 191–204 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

