Brain Renin-Angiotensin System and Microglial Polarization: Implications for Aging and Neurodegeneration
- PMID: 28515690
- PMCID: PMC5413566
- DOI: 10.3389/fnagi.2017.00129
Brain Renin-Angiotensin System and Microglial Polarization: Implications for Aging and Neurodegeneration
Abstract
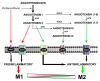
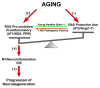
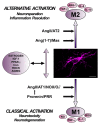
Microglia can transform into proinflammatory/classically activated (M1) or anti-inflammatory/alternatively activated (M2) phenotypes following environmental signals related to physiological conditions or brain lesions. An adequate transition from the M1 (proinflammatory) to M2 (immunoregulatory) phenotype is necessary to counteract brain damage. Several factors involved in microglial polarization have already been identified. However, the effects of the brain renin-angiotensin system (RAS) on microglial polarization are less known. It is well known that there is a "classical" circulating RAS; however, a second RAS (local or tissue RAS) has been observed in many tissues, including brain. The locally formed angiotensin is involved in local pathological changes of these tissues and modulates immune cells, which are equipped with all the components of the RAS. There are also recent data showing that brain RAS plays a major role in microglial polarization. Level of microglial NADPH-oxidase (Nox) activation is a major regulator of the shift between M1/proinflammatory and M2/immunoregulatory microglial phenotypes so that Nox activation promotes the proinflammatory and inhibits the immunoregulatory phenotype. Angiotensin II (Ang II), via its type 1 receptor (AT1), is a major activator of the NADPH-oxidase complex, leading to pro-oxidative and pro-inflammatory effects. However, these effects are counteracted by a RAS opposite arm constituted by Angiotensin II/AT2 receptor signaling and Angiotensin 1-7/Mas receptor (MasR) signaling. In addition, activation of prorenin-renin receptors may contribute to activation of the proinflammatory phenotype. Aged brains showed upregulation of AT1 and downregulation of AT2 receptor expression, which may contribute to a pro-oxidative pro-inflammatory state and the increase in neuron vulnerability. Several recent studies have shown interactions between the brain RAS and different factors involved in microglial polarization, such as estrogens, Rho kinase (ROCK), insulin-like growth factor-1 (IGF-1), tumor necrosis factor α (TNF)-α, iron, peroxisome proliferator-activated receptor gamma, and toll-like receptors (TLRs). Metabolic reprogramming has recently been involved in the regulation of the neuroinflammatory response. Interestingly, we have recently observed a mitochondrial RAS, which is altered in aged brains. In conclusion, dysregulation of brain RAS plays a major role in aging-related changes and neurodegeneration by exacerbation of oxidative stress (OS) and neuroinflammation, which may be attenuated by pharmacological manipulation of RAS components.
Keywords: NADPH-oxidase; Nox; Parkinson; angiotensin; microglia; neuroinflammation; neuroprotection; oxidative stress.
Figures

References
-
- Alberici L. C., Oliveira H. C., Paim B. A., Mantello C. C., Augusto A. C., Zecchin K. G., et al. . (2009). Mitochondrial ATP-sensitive K+ channels as redox signals to liver mitochondria in response to hypertriglyceridemia. Free Radic. Biol. Med. 47, 1432–1439. 10.1016/j.freeradbiomed.2009.08.013 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

