An Analysis of Trafficking Receptors Shows that CD44 and P-Selectin Glycoprotein Ligand-1 Collectively Control the Migration of Activated Human T-Cells
- PMID: 28515724
- PMCID: PMC5413510
- DOI: 10.3389/fimmu.2017.00492
An Analysis of Trafficking Receptors Shows that CD44 and P-Selectin Glycoprotein Ligand-1 Collectively Control the Migration of Activated Human T-Cells
Abstract
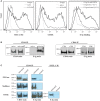
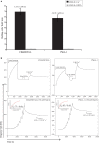
Selectins guide the traffic of activated T-cells through the blood stream by mediating their tethering and rolling onto inflamed endothelium, in this way acting as beacons to help navigate them to sites of inflammation. Here, we present a comprehensive analysis of E-selectin ligands expressed on activated human T-cells. We identified several novel glycoproteins that function as E-selectin ligands. Specifically, we compared the role of P-selectin glycoprotein ligand-1 (PSGL-1) and CD43, known E-selectin ligands, to CD44, a ligand that has not previously been characterized as an E-selectin ligand on activated human T-cells. We showed that CD44 acts as a functional E-selectin ligand when expressed on both CD4+ and CD8+ T-cells. Moreover, the CD44 protein carries a binding epitope identifying it as hematopoietic cell E- and/or L-selectin ligand (HCELL). Furthermore, by knocking down these ligands individually or together in primary activated human T-cells, we demonstrated that CD44/HCELL, and not CD43, cooperates with PSGL-1 as a major E-selectin ligand. Additionally, we demonstrated the relevance of our findings to chronic autoimmune disease, by showing that CD44/HCELL and PSGL-1, but not CD43, from T-cells isolated from psoriasis patients, bind E-selectin.
Keywords: CD44; E-selectin; P-selectin glycoprotein ligand-1 (CD162); cell adhesion; cell migration; hematopoietic cell E- and/or L-selectin ligand; human activated T-cells; psoriasis.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

