Cisplatin-based chemoradiotherapy with 5-fluorouracil or pemetrexed in patients with locally advanced, unresectable esophageal squamous cell carcinoma: A retrospective analysis
- PMID: 28515926
- PMCID: PMC5431244
- DOI: 10.3892/mco.2017.1222
Cisplatin-based chemoradiotherapy with 5-fluorouracil or pemetrexed in patients with locally advanced, unresectable esophageal squamous cell carcinoma: A retrospective analysis
Abstract
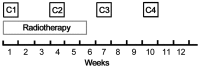
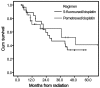
Treatment with 5-fluorouracil (5-FU) and cisplatin (PF regimen) remains the most frequently used chemotherapy for esophageal squamous cell carcinoma (SCC). The aim of the present study was to assess the efficacy and safety of pemetrexed/cisplatin (PP regimen) as definitive treatment compared with PF. A total of 60 patients with locally advanced, unresectable SCC of the esophagus receiving concomitant chemoradiotherapy were recruited in this study; of those patients, 29 received four cycles (two concomitant and two post-radiotherapy) of the PF regimen (arm A, cisplatin 25 mg/m2/day i.v. on days 1-3 plus 5-FU 800 mg/m2/24 h by continuous infusion on days 1-5) and 31 received four cycles of the PP regimen (arm B, cisplatin 25 mg/m2/day i.v. on days 1-3 plus pemetrexed 500 mg/m2 on day 1). All the patients in both arms received a total radiation dose of 59.6 Gy. The two arms were well-matched for age, gender, Karnofsky performance status, TNM stage, tumor location and length. The overall response rate was 89.7% in arm A vs. 93.5% in arm B (P>0.05). The median overall survival was 26.1 months [95% confidence interval (CI): 15.3-36.8 months] in arm A vs. 28.7 months (95% CI: 9.4-48.0 months) in arm B (P>0.05). Severe esophagitis occurred in 31.0% (9/29) of the patients in arm A vs. 12.9% (4/31) of the patients in arm B; the difference was statistically significant (P=0.036). Grade 3/4 leukopenia and thrombocytopenia occurred in 4 (13.8%) and 1 (3.4%) patients, respectively, in arm A vs. 12 (38.7%) and 6 (19.4%) patients, respectively, in arm B; the differences were statistically significant (P=0.029 and 0.041, respectively). Therefore, chemoradiotherapy with the PP regimen achieved therapeutic results comparable with those of the PF regimen; in terms of toxicity, the incidence of hematological toxicity was higher and that of esophagitis was lower with the PP regimen.
Keywords: 5-fluorouracil; chemoradiotherapy; esophageal; pemetrexed; squamous cell carcinoma.
Figures
References
-
- Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA, Jr, Al-Sarraf M, Byhardt R, Russell AH, Beitler JJ, Spencer S, et al. Chemoradiotherapy of locally advanced esophageal cancer: Long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623–1627. doi: 10.1001/jama.281.17.1623. - DOI - PubMed
-
- Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, Okawara G, Rosenthal SA, Kelsen DP. INT 0123 (Radiation Therapy Oncology Group 94–05) phase III trial of combined-modality therapy for esophageal cancer: High-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167–1174. doi: 10.1200/JCO.20.5.1167. - DOI - PubMed
-
- Kato K, Muro K, Minashi K, Ohtsu A, Ishikura S, Boku N, Takiuchi H, Komatsu Y, Miyata Y, Fukuda H. Gastrointestinal Oncology Study Group of the Japan Clinical Oncology Group (JCOG): Phase II study of chemoradiotherapy with 5-fluorouracil and cisplatin for Stage II–III esophageal squamous cell carcinoma: JCOG trial (JCOG 9906) Int J Radiat Oncol Biol Phys. 2011;81:684–690. doi: 10.1016/j.ijrobp.2010.06.033. - DOI - PubMed
-
- Zhao KL, Shi XH, Jiang GL, Yao WQ, Guo XM, Wu GD, Zhu LX. Late course accelerated hyperfractionated radiotherapy plus concurrent chemotherapy for squamous cell carcinoma of the esophagus: A phase III randomized study. Int J Radiat Oncol Biol Phys. 2005;62:1014–1020. doi: 10.1016/j.ijrobp.2004.12.022. - DOI - PubMed
-
- Higuchi K, Koizumi W, Tanabe S, Sasaki T, Katada C, Ishiyama H, Hayakawa K. A phase I trial of definitive chemoradiotherapy with docetaxel, cisplatin, and 5-fluorouracil (DCF-R) for advanced esophageal carcinoma: Kitasato digestive disease & oncology group trial (KDOG 0501) Radiother Oncol. 2008;87:398–404. doi: 10.1016/j.radonc.2008.03.006. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous