Evaluation of dosing strategy for pembrolizumab for oncology indications
- PMID: 28515943
- PMCID: PMC5433037
- DOI: 10.1186/s40425-017-0242-5
Evaluation of dosing strategy for pembrolizumab for oncology indications
Abstract
Background: Traditionally, most monoclonal antibodies (mAbs) have been dosed based on body weight because of perceived contribution of body size in pharmacokinetic variability. The same approach was used in the initial pembrolizumab studies; however, following availability of PK data, the need for weight-based dosing for pembrolizumab was reassessed.
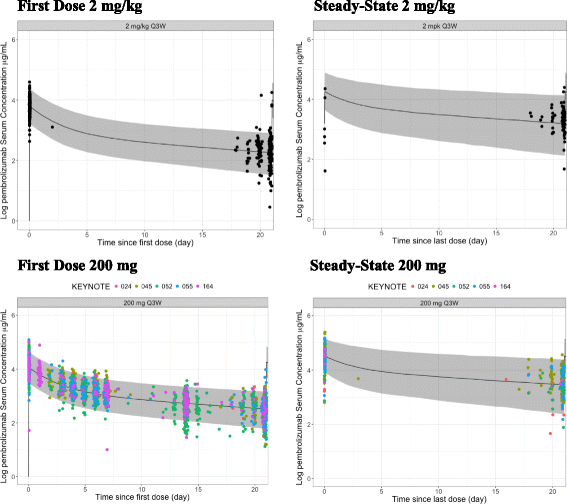
Methods: A previously established population PK (popPK) model as well as exposure-response results from patients with advanced melanoma or non-small cell lung cancer (NSCLC) were used to evaluate the potential application of a fixed dosing regimen with the aim of maintaining pembrolizumab exposures within the range demonstrated to provide near maximal efficacy and acceptable safety. Individual PK exposures for the selected fixed dosing regimen from recently completed trials with head and neck cancer, NSCLC, microsatellite instability high (MSI-H) in colorectal cancer (CRC) and urothelial cancer were used to confirm acceptability. To determine whether fixed dosing would maintain exposures within the range of clinical experience, the individual AUC distributions with fixed dosing were compared with the range of exposures from the pembrolizumab doses that were evaluated in early studies (2 mg/kg Q3W, 10 mg/kg Q3W/Q2W).
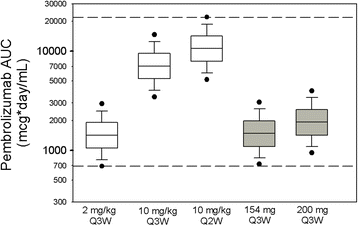
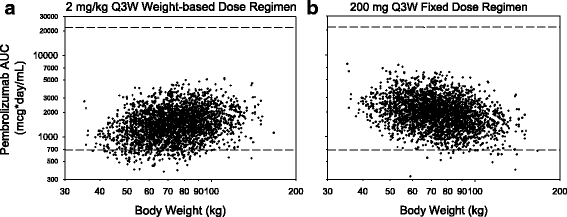
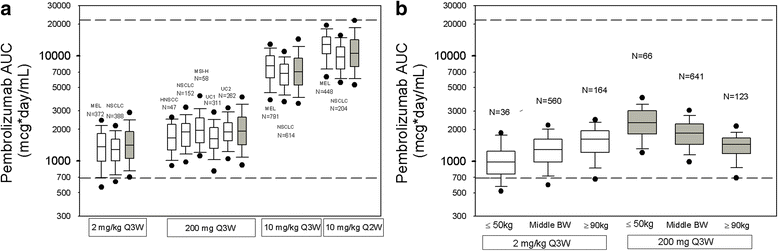
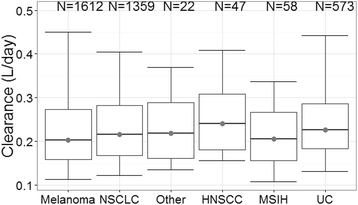
Results: Body-weight dependence of clearance was characterized by a power relationship with an exponent of 0.578, a value consistent with fixed- and weight-based dosing providing similar control of PK variability. A fixed dose of 200 mg Q3W was investigated in trials based on predicted exposures maintained within the established exposure range in all patients. Mean (% CV, n) AUCss, 6-weeks was 1.87 (37%, 830), 1.38 (38%, 760) and 7.63 (35%, 1405) mg*day/mL in patients receiving 200 mg, 2 mg/kg and 10 mg/kg Q3W pembrolizumab. High-weight patients had the lowest exposures with 200 mg Q3W; however, exposures in this group (>90 kg) were within the range of prior clinical experience at 2 mg/kg Q3W associated with near maximal efficacy.
Conclusions: Doses of 200 mg and 2 mg/kg provide similar exposure distributions with no advantage to either dosing approach with respect to controlling PK variability. These findings suggest that weight-based and fixed-dose regimens are appropriate for pembrolizumab.
Keywords: Clinical dose; Dosing strategy; Exposure-response analysis; Fixed dose; Flat dose; Pembrolizumab; Population pharmacokinetics analysis.
Figures
References
-
- Ahamadi M, Freshwater T, Prohn M, Li CH, De Alwis DP, De Greef R, et al. Model-based characterization of the pharmacokinetics of pembrolizumab, a humanized anti–Pd-1 monoclonal antibody, in advanced solid tumors. CPT Pharmacometrics Syst Pharmacol. 2017;6(1):49–57. doi: 10.1002/psp4.12139. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
