Transplantation of Human Embryonic Stem Cell-Derived Retinal Cells into the Subretinal Space of a Non-Human Primate
- PMID: 28516002
- PMCID: PMC5433804
- DOI: 10.1167/tvst.6.3.4
Transplantation of Human Embryonic Stem Cell-Derived Retinal Cells into the Subretinal Space of a Non-Human Primate
Abstract
Purpose: Previous studies have demonstrated the ability of retinal cells derived from human embryonic stem cells (hESCs) to survive, integrate into the host retina, and mediate light responses in murine mouse models. Our aim is to determine whether these cells can also survive and integrate into the retina of a nonhuman primate, Saimiri sciureus, following transplantation into the subretinal space.
Methods: hESCs were differentiated toward retinal neuronal fates using our previously published technique and cultured for 60 to 70 days. Differentiated cells were further treated with 20 μM N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT) for a period of 5 days immediately prior to subretinal transplantation. Differentiated cells were labeled with a lentivirus expressing GFP. One million cells (10,000 cells/μL) were injected into the submacular space into a squirrel monkey eye, using an ab externo technique.
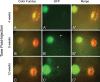
Results: RetCam imaging demonstrated the presence and survival of human donor cells 3 months after transplantation in the S. sciureus eye. Injected cells consolidated in the temporal macula. GFP+ axonal projections were observed to emanate from the central consolidation of cells at 1 month, with some projecting into the optic nerve by 3 months after transplantation.
Conclusions: Human ES cell-derived retinal neurons injected into the submacular space of a squirrel monkey survive at least 3 months postinjection without immunosuppression. Some donor cells appeared to integrate into the host inner retina, and numerous donor axonal projections were noted throughout, with some projecting into the optic nerve.
Translational relevance: These data illustrate the feasibility of hESC-derived retinal cell replacement in the nonhuman primate eye.
Keywords: AMD; glaucoma; primate; retina; stem cell transplantation.
Figures





References
-
- Klein R,, Chou CF,, Klein BE,, Zhang X,, Meuer SM,, Saaddine JB. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol. 2011; 129: 75–80. - PubMed
-
- Banin E,, Obolensky A,, Idelson M,, et al. Retinal incorporation and differentiation of neural precursors derived from human embryonic stem cells. Stem Cells. 2006; 24: 246–257. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

