Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis
- PMID: 28516167
- PMCID: PMC5431585
- DOI: 10.5301/jsrd.5000231
Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis
Abstract
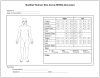
The modified Rodnan skin score (mRSS) is a measure of skin thickness and is used as a primary or secondary outcome measure in clinical trials of systemic sclerosis (scleroderma). This state-of-art review provides a historical perspective of the development of the mRSS, summarizes the performance of mRSS as an outcome measure, provides guidance on assessing mRSS, and makes recommendations for incorporation of the mRSS into clinical trials.
Conflict of interest statement
CONFLICTS Dr. Khanna has consultancy relationships with Actelion, Bayer, Bristol-Myers Squibb, ChemomAb, Corbus, Cytori, Eicos, EMD Serono, Genentech/Roche, Gilead, Glaxo SmithKline, MedImmune, Medac, and Sanofi-Aventis/Genzyme. He has received research funding from Astra-Zeneca, Bayer, Bristol-Myers Squibb, and Pfizer. This work was funded in part by the NIH/NIAMS K24 AR06312 grant. Dr. Furst has consultancy relationships and/or has received research funding in relationship grant/research from Amgen, BMS, NIH, Novartis, Pfizer, Roche/Genentech, AbbVie, Amgen, BMS, Cytori, Janssen, NIH, Novartis, Pfizer, Roche/Genentech, and UCB. Dr. Clements has no relevant conflicts. Dr. Allanore has consultancy relationships and/or has received research funding in relationship with the treatment of systemic sclerosis from Actelion, Bayer, Biogen Idec, Bristol-Myers Squibb, Genentech/Roche, Inventiva, Medac, Pfizer, Sanofi/Genzyme, Servier and UCB. Dr. Baron has no relevant conflicts. Dr. Czirjak has consultancy relationships with the treatment of systemic sclerosis from Actelion, Bayer, Genentech/Roche, Inventiva, Medac, and Pfizer. Dr. Distler has/had consulting relationships from 4 D Science, Actelion, Active Biotec, Chemom AG, Bayer, BiogenIdec, BMS, Boehringer Ingelheim, ChemomAb, EpiPharm, espeRare foundation, Genentech/Roche, GSK, Inventiva, Lilly, medac, Mepha, MedImmune, Pharmacyclics, Pfizer, Sanofi, Serodapharm, and Sinoxa; and grants from Actelion, Bayer, Boehringer Ingelheim, Pfizer, and Sanofi. Patent licensed: mir-29 for the treatment of systemic sclerosis. Speaker fees from AbbVie, iQone Healthcare, Mepha Dr. Foeldvari has consultancy relationships with Bayer, Roche-Genentech, Novartis, Abbive, Chugai. Speakers fee: Pfizer, Abbvie, and Medac Dr. Kuwana has consultancy or speaker fees from Bayer, Boehringer Ingelheim, Chugai, Actelion, and Glaxo SmithKline, and/or has received research funding from Chugai, Actelion, and Bayer. Dr. Matucci-Cerinic has active consultancies or has received grant support from Amgen, BMS, Pfizer, Bruno farma, and Actelion. Consultant: BMS, Actelion, Pfizer, Inventiva, Chemomab Dr. Mayes has consultancy relationship with and/or has received research funding from Bayer, Boehringer-Ingelheim, Corbus, Cytori, Genentech/Roche. Dr. Medsger has consultancy relationship with iBio. Dr. Merkel has consultancy relationship with and/or has received research funding from Actelion, Bristol-Myers Squibb, ChemoCentryx, Genentech/Roche, Glaxo SmithKline, Kypha, MedImmune/AztraZeneca, PrincipoBio, Sanofi-Aventis/Genzyme. Dr. Pope has consultancy relationship with AbbVie, Actelion, Amgen, Bayer, BMS, Hospira, Janssen, Lilly, Merck, Novartis, Pfizer, Roche, Sanofi, UCB. Dr. Seibold has consultancy relationship with Anthera, Bayer, BioGen IDEC, Blade Therapeutics, Boehringer Ingelheim, Covis Pharma, Cytori, Eiger, EMD Serono, Milestone, Mitsubishi, Sanofi-Aventis, Teva and Xenikos. There are no speaker’s bureaus or stock holdings. Dr. Steen has consultancy fees from Bristol Myers Squibb, Cytori, Bayer, Gilead, and research grant funding from CSL Behring, United Therapeutics and Gilead, and Clinical Trial research funding from Cytori, Roche, Boehringer Ingelheim, EMD Serono, Covis, and Reata Dr. Stevens has consultancy relationship or received research funding form Actelion, GSK, Bayer and Pfizer and has received speakers fees from Actelion and GSK Dr. Denton has consultancy relationships with or speaker fees from Actelion, Bristol-Myers Squibb, GlaxoSmithKline, Bayer, Sanofi-Aventis, Boehringer Ingelheim, Genentech-Roche, and CSL Behring, and research grant funding from GlaxoSmithKline, Actelion, Novartis, CSL Behring.
Figures







References
-
- LeRoy EC, Medsger TA., Jr Criteria for the classification of early systemic sclerosis. J Rheumatol. 2001;28(7):1573–6. Epub 2001/07/27. - PubMed
-
- Perera A, Fertig N, Lucas M, Rodriguez-Reyna TS, Hu P, Steen VD, Medsger TA., Jr Clinical subsets, skin thickness progression rate, and serum antibody levels in systemic sclerosis patients with anti-topoisomerase I antibody. Arthritis and rheumatism. 2007;56(8):2740–6. doi: 10.1002/art.22747. - DOI - PubMed
-
- Shand L, Lunt M, Nihtyanova S, Hoseini M, Silman A, Black CM, Denton CP. Relationship between change in skin score and disease outcome in diffuse cutaneous systemic sclerosis: application of a latent linear trajectory model. Arthritis Rheum. 2007;56(7):2422–31. doi: 10.1002/art.22721. - DOI - PubMed
-
- Clements PJ, Wong WK, Hurwitz EL, Furst DE, Mayes M, White B, Wigley F, Weisman M, Barr W, Moreland L, Medsger TA, Jr, Steen V, Martin RW, Collier D, Weinstein A, Lally E, Varga J, Weiner SR, Andrews B, Abeles M, Seibold JR. The Disability Index of the Health Assessment Questionnaire is a predictor and correlate of outcome in the high-dose versus low-dose penicillamine in systemic sclerosis trial. Arthritis Rheum. 2001;44(3):653–61. doi: 10.1002/1529-0131(200103)44:3<653::AID-ANR114>3.0.CO;2-Q. - DOI - PubMed
-
- Clements PJ, Hurwitz EL, Wong WK, Seibold JR, Mayes M, White B, Wigley F, Weisman M, Barr W, Moreland L, Medsger TA, Jr, Steen VD, Martin RW, Collier D, Weinstein A, Lally E, Varga J, Weiner SR, Andrews B, Abeles M, Furst DE. Skin thickness score as a predictor and correlate of outcome in systemic sclerosis: high-dose versus low-dose penicillamine trial. Arthritis Rheum. 2000;43(11):2445–54. doi: 10.1002/1529-0131(200011)43:11<2445::AID-ANR11>3.0.CO;2-Q. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
