Nasal decontamination for the prevention of surgical site infection in Staphylococcus aureus carriers
- PMID: 28516472
- PMCID: PMC6481881
- DOI: 10.1002/14651858.CD012462.pub2
Nasal decontamination for the prevention of surgical site infection in Staphylococcus aureus carriers
Abstract
Background: Surgical site infection rates in the month following surgery vary from 1% to 5%. Due to the large number of surgical procedures conducted annually, the costs of these surgical site infections (SSIs) can be considerable in financial and social terms. Nasal decontamination using antibiotics or antiseptics is performed to reduce the risk of SSIs by preventing organisms from the nasal cavity being transferred to the skin where a surgical incision will be made. Staphylococcus aureus (S aureus) colonises the nasal cavity and skin of carriers and can cause infection in open or unhealed surgical wounds. S aureus is the leading nosocomial (hospital-acquired) pathogen in hospitals worldwide. The potential effectiveness of nasal decontamination of S aureus is thought to be dependent on both the antibiotic/antiseptic used and the dose of application; however, it is unclear whether nasal decontamination actually reduces postoperative wound infection in S aureus carriers.
Objectives: To assess the effects of nasal decontamination on preventing surgical site infections (SSIs) in people who are S aureus carriers undergoing surgery.
Search methods: In September 2016 we searched the Cochrane Wounds Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library), Ovid MEDLINE, Ovid MEDLINE (In-Process & Other Non-Indexed Citations), Ovid Embase, and EBSCO CINAHL Plus. We also searched three clinical trial registries and the references of included studies and relevant systematic reviews. There were no restrictions based on language, date of publication or study setting.
Selection criteria: Randomised controlled trials (RCTs) which enrolled S aureus carriers with any type of surgery and assessed the use of nasal decontamination with antiseptic/antibiotic properties were included in the review.
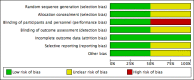
Data collection and analysis: Two review authors independently performed study selection, data extraction, risk of bias assessment and GRADE assessment.
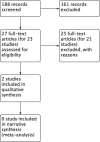
Main results: We located two studies (291 participants) for inclusion in this review. The trials were clinically heterogeneous with differences in duration of follow-up, and nasal decontamination regimens. One study compared mupirocin (2% contained in a base of polyethylene glycol 400 and polyethylene glycol 3350) with a placebo in elective cardiac surgery patients; and one study compared Anerdian (iodine 0.45% to 0.57% (W/V), chlorhexidine acetate 0.09% to 0.11% (W/V)) with no treatment also in cardiac surgery patients. The trials reported limited outcome data on SSI, adverse events and secondary outcomes (e.g. S aureus SSI, mortality). Mupirocin compared with placeboThis study found no clear difference in SSI risk following use of mupirocin compared with placebo (1 trial, 257 participants); risk ratio (RR) 1.60, 95% confidence interval (CI) 0.79 to 3.25 based on 18/130 events in the mupirocin group and 11/127 in the control group; low-certainty evidence (downgraded twice due to imprecision). Anerdian compared with no treatmentIt is uncertain whether there is a difference in SSI risk following treatment with Anerdian compared with no treatment (1 trial, 34 participants); RR 0.89, 95% CI 0.06 to 13.08 based on 1/18 events in the Anerdian group and 1/16 in the control group; very low certainty evidence (downgraded twice due to imprecision and once due to risk of bias).
Authors' conclusions: There is currently limited rigorous RCT evidence available regarding the clinical effectiveness of nasal decontamination in the prevention of SSI. This limitation is specific to the focused question our review addresses, looking at nasal decontamination as a single intervention in participants undergoing surgery who are known S aureus carriers. We were only able to identify two studies that met the inclusion criteria for this review and one of these was very small and poorly reported. The potential benefits and harms of using decontamination for the prevention of SSI in this group of people remain uncertain.
Conflict of interest statement
Zhenmi Liu: my employment at the University of Manchester is funded by the NIHR (NIHR Research Methods Programme Systematic Review Fellowship NIHR‐RMFI‐2015‐06‐52).
Gill Norman: my employment at the University of Manchester is funded by the NIHR and focuses on high priority Cochrane reviews in the prevention and treatment of wounds.
Zipporah Iheozor‐Ejiofor: none known.
Jason KF Wong: none known.
Emma J Crosbie: I am a Scientific Editor for BJOG. I have received funding from an NIHR Clinician Scientist Award, the HTA, Wellbeing of Women/the Wellcome Trust and Central Manchester University Hospitals NHS Foundation Trust. I am an employee of the University of Manchester.
Peter Wilson: I am a consultant microbiologist in the NHS advising on antibiotic use and I advise some private hospitals on infection control. I am a member of a clinical trial drug safety monitoring board for a monoclonal antibody. I have been an expert witness in infection related cases. I hold a number of non‐commercial grants for research in the area of transmission of infection. I am part funded by the UCLH/UCL Comprehensive Biomedical Centre with funding from the Department of Health's NIHR Biomedical Research Centres.
Figures



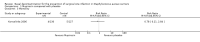

Update of
References
References to studies included in this review
Konvalinka 2006 {published data only}
Yu 2011 {published data only}
-
- Yu M, Mao J, Sun J, Yuan Z. Relationship between nasal colonization of Staphylococcus aureus and nosocomial infection after cardiac surgery. Journal of Shanghai Jiaotong University 2011;31(4):473‐6.
References to studies excluded from this review
Andenaes 1996 {published data only}
-
- Andenaes K, Lingaas E, Amland PF, Giercksky K‐E, Abyholm F. Preoperative bacterial colonization and its influence on postoperative wound infections in plastic surgery. Journal of Hospital Infection 1996;34(4):291‐9. - PubMed
Anonymous 2004 {published data only}
-
- Anonymous. Summaries for patients. Effects of antibiotic nose ointment for hospitalized patients with Staphylococcus aureus. Annals of Internal Medicine 2004;140(6):136. - PubMed
Bode 2010 {published data only}
-
- Bode LG, Kluytmans JA, Wertheim HF, Bogaers D, Vandenbroucke‐Grauls CM, Roosendaal R, et al. Preventing surgical‐site infections in nasal carriers of Staphylococcus aureus. New England Journal of Medicine 2010;362(1):9‐17. - PubMed
Caelli 2000 {published data only}
-
- Caelli M, Porteous J, Carson CF, Heller R, Riley TV. Tea tree oil as an alternative topical decolonization agent for methicillin‐resistant Staphylococcus aureus. Journal of Hospital Infection 2000;46(3):236‐7. - PubMed
Dryden 2004 {published data only}
-
- Dryden MS, Dailly S, Crouch M. A randomized, controlled trial of tea tree topical preparations versus a standard topical regimen for the clearance of MRSA colonization. Journal of Hospital Infection 2004;56(4):283‐6. - PubMed
Garcia 2003 {published data only}
-
- García AM, Villa MV, Escudero ME, Gómez P, Vélez MM, Múnera MI, et al. Use of nasal mupirocin for Staphylococcus aureus: effect on nasal carriers and nosocomial infections. Biomédica 2003;23(2):173‐9. - PubMed
Golan 2010 {published data only}
-
- Golan Y. Decolonization of nostrils and skin of nasal carriers of S. aureus at admission prevented hospital‐associated infection. Annals of Internal Medicine 2010;152(10):JC5‐9. - PubMed
Harbarth 2000 {published data only}
-
- Harbarth S, Liassine N, Dharan S, Herrault P, Auckenthaler R, Pittet D. Risk factors for persistent carriage of methicillin‐resistant Staphylococcus aureus. Clinical Infectious Diseases 2000;31(6):1380‐5. - PubMed
Jabbour 2010 {published data only}
-
- Jabbour H, Madi‐Jebara S, Jabbour K, Yazigi A, Haddad F, Hayek G, et al. Does nasal decontamination reduce the incidence of infections after cardiac surgery?. The Lebanese Medical Journal 2010;58(2):65‐70. - PubMed
Kalmeijer 2002 {published data only}
-
- Kalmeijer MD, Coertjens H, Baere GA, Stuurman A, Belkum A, Kluytmans JA. Postoperative wound infections in orthopedic surgery. The effect of mupirocin nasal ointment. Pharmaceutisch Weekblad 2001;136(29):730‐1.
-
- Kalmeijer MD, Coertjens H, Baere GA, Stuurman A, Belkum A, Kluytmans JA. Surgical site infections in orthopedic surgery: the effect of mupirocin nasal ointment in a double‐blind, randomized, placebo‐controlled study. Clinical Infectious Diseases 2002;35(4):353‐8. - PubMed
Mehta 2013 {published data only}
-
- Mehta MS, Hacek DM, Kufner BA, Price C, Peterson LR. Dose‐ranging study to assess the application of intranasal 2% mupirocin calcium ointment to eradicate Staphylococcus aureus nasal colonization. Surgical Infections 2013;14(1):69‐72. - PubMed
Moon 2010 {published data only}
-
- Moon KT. Reducing hospital‐associated infections in Staphylococcus aureus carriers. American Family Physician 2010;82(5):528.
Perl 2002 {published data only}
-
- Perl TM, Cullen JJ, Wenzel RP, Zimmerman MB, Pfaller MA, Sheppard D, et al. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. New England Journal of Medicine 2002;346(24):1871‐7. - PubMed
Phillips 2014 {published data only}
Rijen 2012 {published data only}
Segers 2006 {published data only}
-
- Segers P, Speekenbrink RG, Ubbink DT, Ogtrop ML, Mol BA. Prevention of nosocomial infection in cardiac surgery by decontamination of the nasopharynx and oropharynx with chlorhexidine gluconate: a randomized controlled trial. JAMA 2006;296(20):2460‐6. - PubMed
-
- Segers P, Speekenbrink RG, Ubbink DT, Ogtrop ML, Mol BA. Prevention of nosocomial infections after cardiac surgery by decontamination of the nasopharynx and oropharynx with chlorhexidine; a prospective, randomised study. Nederlands Tijdschrift voor Geneeskunde 2008;152(13):760‐7. - PubMed
Shrem 2016 {published data only}
-
- Egozi T, Shrem G, Naeh A, Hallak M, Walfisch A. Nasal carriage of Staphylococcus aureus in patients undergoing cesarean section and surgical site infection: a prospective randomized trial. American Journal of Obstetrics and Gynecology 2015;212(1 (Suppl)):S206‐7.
-
- Shrem G, Egozi T, Naeh A, Hallak M, Walfisch A. Pre‐cesarean Staphylococcus aureus nasal screening and decolonization: a prospective randomized controlled trial. Journal of Maternal‐Fetal and Neonatal Medicine 2016;29(23):3906‐11. - PubMed
Shuman 2012 {published data only}
-
- Shuman AG, Shuman EK, Hauff SJ, Fernandes LL, Light E, Chenoweth CE, et al. Preoperative topical antimicrobial decolonization in head and neck surgery. Laryngoscope 2012;122(11):2454‐60. - PubMed
Sousa 2016 {published data only}
-
- Sousa RJ, Barreira PM, Leite PT, Santos AC, Ramos MH, Oliveira AF. Preoperative Staphylococcus aureus screening/decolonization protocol before total joint arthroplasty: results of a small prospective randomized trial. Journal of Arthroplasty 2016;31(1):234‐9. - PubMed
Tai 2013 {published data only}
-
- Tai YJ, Borchard KL, Gunson TH, Smith HR, Vinciullo C. Nasal carriage of Staphylococcus aureus in patients undergoing Mohs micrographic surgery is an important risk factor for postoperative surgical site infection: a prospective randomised study. Australasian Journal of Dermatology 2013;54(2):109‐14. - PubMed
-
- Tai YJ, Gunson TH, Borchard KL, Smith HR, Vinciullo C. A prospective randomised study of Staphylococcus aureus nasal carriage as a major risk factor for infection in Mohs micrographic surgery. Australasian Journal of Dermatology 2012;53(4):A8. - PubMed
Vinciullo 2014 {published data only}
-
- Vinciullo C. Antimicrobial stewardship and surgical site infection: an evidence based approach. Australasian Journal of Dermatology 2014;55(S1):12‐3.
Additional references
Allegranzi 2010
-
- Allegranzi B, Nejad SB, Combescure C, Graafmans W, Attar H, Donaldson L, et al. Burden of endemic health‐care‐associated infection in developing countries: systematic review and meta‐analysis. Lancet 2010;377(9761):228‐41. - PubMed
Anderson 2008
-
- Anderson DJ, Chen LF, Schmader KE, Sexton DJ, Choi Y, Link K, et al. Poor functional status as a risk factor for surgical site infection due to methicillin‐resistant staphylococcus aureus. Infection Control and Hospital Epidemiology 2008;29(9):832‐9. - PubMed
Astagneau 2009
-
- Astagneau P, L'Hériteau F, Daniel F, Parneix P, Venier AG, Malavaud S, et al. ISO‐RAISIN Steering Group. Reducing surgical site infection incidence through a network: results from the French ISO‐RAISIN surveillance system. Journal of Hospital Infection 2009;72(2):127‐34. - PubMed
Awad 2012
-
- Awad SS. Adherence to surgical care improvement project measures and post‐operative surgical site infections. Surgical Infections 2012;13(2):234‐7. - PubMed
Bebko 2015
-
- Bebko SP, Green DM, Awad SS. Effect of a preoperative decontamination protocol on surgical site infections in patients undergoing elective orthopedic surgery with hardware implantation. JAMA Surgery 2015;150(5):390‐5. - PubMed
British National Formulary 2016
-
- British Medical Association, Royal Pharmaceutical Society. British National Formulary: Naseptin® (Alliance). www.evidence.nhs.uk/formulary/bnf/current/12‐ear‐nose‐and‐oropharynx/122... (accessed 19 September 2016).
Brown 2014
Bruce 2001
-
- Bruce J, Russell EM, Mollison J, Krukowski ZH. The measurement and monitoring of surgical adverse events. Health Technology Assessment 2001;5(22):1‐194. - PubMed
Button 2010
-
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nature Reviews. Neuroscience 2010;14(5):365‐76. - PubMed
Campoccia 2013
-
- Campoccia D, Montanaro L, Arciola CR. A review of the clinical implications of anti‐infective biomaterials and infection‐resistant surfaces. Biomaterials 2013;34(33):8018‐29. - PubMed
CDC 2017
-
- Centers for Disease Control and Prevention (CDC). Surgical site infection (SSI) event. Procedure‐associated module. January 2017. www.cdc.gov/nhsn/PDFs/pscmanual/9pscssicurrent.pdf (accessed 20 January 2017).
Chemaly 2010
-
- Chemaly RF, Hachem RY, Husni RN, Bahna B, Abou Rjaili G, Waked A, et al. Characteristics and outcomes of methicillin resistant staphylococcus aureus surgical‐site infections in patients with cancer: a case‐control study. Annals of Surgical Oncology 2010;17(6):1499‐506. - PubMed
Coates 2009
de Lissovoy 2009
-
- Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. American Journal of Infection Control 2009;37(5):387‐97. - PubMed
Deeks 2011
-
- Deeks JJ, Higgins JP, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Edwards 2008
-
- Edwards C, Counsell A, Boulton C, Moran CG. Early infection after hip fracture surgery: risk factors, costs and outcome. Journal of Bone and Joint Surgery 2008;90(6):770‐7. - PubMed
Gibbons 2011
-
- Gibbons C, Bruce J, Carpenter J, Wilson AP, Wilson J, Pearson A, et al. Identification of risk factors by systematic review and development of risk‐adjusted models for surgical site infection. Health Technology Assessment 2011;15(30):1‐156. - PubMed
GRADE 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, GRADE working group. GRADE Handbook. Updated October 2013. gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbo... (accessed 5 August 2016).
Hibbard 2002
-
- Hibbard JS, Mulbery GK, Brady AR. A clinical study comparing the skin antisepsis and safety of chloraprep, 70% isopropylalcohol and 2% aqueous chlorhexidine. Journal of Infusion Nursing 2002;25(4):244‐9. - PubMed
HICPAC 1999
-
- Hospital Infection Control Practices Advisory Committee (HICPAC). Guideline for prevention of surgical site infection 1999. Infection Control and Hospital Epidemiology 1999;20(4):259. - PubMed
Higgins 2003
Higgins 2011a
-
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JP, Deeks JJ, Altman DG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hoffrage 2000
-
- Hoffrage U, Lindsey S, Hertwig R, Gigerenzer G. Medicine: communicating statistical information. Science 2000;290(5500):2261‐2. - PubMed
Jenks 2014
-
- Jenks PJ, Laurent M, McQuarry S, Watkins R. Clinical and economic burden of surgical site infection and predicted financial consequences of elimination of SSI from an English hospital. Journal of Hospital Infection 2014;86(1):24‐33. - PubMed
Kontopantelis 2012
-
- Kontopantelis E, Reeves D. Performance of statistical methods for meta‐analysis when true study effects are non‐normally distributed: a simulation study. Statistical Methods in Medical Research 2012;21(4):409‐26. - PubMed
Kontopantelis 2013
Korol 2013
Leaper 2015
Leekha 2011
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Levy 2013
-
- Levy PY, Ollivier M, Drancourt M, Raoult D, Argenson JN. Relation between nasal carriage of Staphylococcus aureus and surgical site infection in orthopedic surgery: the role of nasal contamination. A systematic literature review and meta‐analysis. Orthopaedics and Traumatology: Surgery and Research 2013;99(6):645‐51. - PubMed
Liberati 2009
Macpherson 2004
-
- Macpherson G, editor. Black's Student Medical Dictionary. London: A&C Black Publishers Limited, 2004.
Mangram 1999
-
- Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection. Infection Control and Hospital Epidemiology 1999;20(4):247‐78. - PubMed
NICE 2008
-
- National Institute for Health and Care Excellence (NICE). Prevention and treatment of surgical site infection. Clinical guideline [CG74]. October 2008. www.nice.org.uk/Guidance/cg74 (accessed 19 September 2016).
Nouwen 2006
Owens 2008
-
- Owens CD, Stoessel K. Surgical site infections: epidemiology, microbiology and prevention. Journal of Hospital Infection 2008;70(S2):3‐10. - PubMed
Patel 2009
-
- Patel JB, Gorwitz RJ, Jernigan JA. Mupirocin resistance. Clinical Infectious Diseases 2009;49(6):935‐41. - PubMed
Peacock 2001
-
- Peacock SJ, Silva I, Lowy FD. What determines nasal carriage of Staphylococcus aureus?. Trends in Microbiology 2001;9(12):605‐10. - PubMed
Plowman 2000
-
- Plowman R, Graves N, Griffin M. The Socio‐Economic Burden of Hospital Acquired Infection. London: Public Health Services Laboratory 2000.
Public Health England 2014
-
- Public Health England. Surveillance of surgical site infections in NHS hospitals in England: 2013 to 2014. www.gov.uk/government/uploads/system/uploads/attachment_data/file/386927... (accessed 1 July 2016).
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schulz 2010
Schünemann 2011a
-
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
-
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
SIGN 2015
-
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. www.sign.ac.uk/methodology/filters.html#random (accessed 10 July 2016).
Smyth 2008
-
- Smyth ET, McIlvenny G, Enstone JE, Emmerson AM, Humphreys H, Fitzpatrick F, et al. Four Country Healthcare Associated Infection Prevalence Survey 2006: overview of the results. Journal of Hospital Infection 2008;69:230‐48. - PubMed
Spann 2004
-
- Spann CT, Taylor SC, Weinberg JM. Topical antimicrobial agents in dermatology. Clinics in Dermatology 2004;50(7):407–21. - PubMed
Sterne 2011
-
- Sterne JA, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Thompson 1999
-
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta‐analysis: a comparison of methods. Statistics in Medicine 1999;18(20):2693‐708. - PubMed
Van Rijen 2008a
Van Rijen 2008b
-
- Rijen MM, Bonten M, Wenzel RP, Kluytmans J. Intranasal mupirocin for reduction of Staphylococcus aureus infections in surgical patients with nasal carriage: a systematic review. Journal of Antimicrobial Chemotherapy 2008;61(2):254‐61. - PubMed
von Eiff 2001
-
- Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. New England Journal of Medicine 2001;344(1):11‐6. - PubMed
Webster 2015
Wertheim 2004
-
- Wertheim HF, Vos MC, Ott A, Belkum A, Voss A, Kluytmans JA, et al. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non‐carriers. Lancet 2004;364(9435):703–5. - PubMed
Wertheim 2005
-
- Wertheim HF, Melles DC, Vos MC, Leeuwen W, Belkum A, Verbrugh HA, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet: Infectious Diseases 2005;5(12):751–62. - PubMed
WHO 2016
-
- World Health Organization (WHO). Global guidelines for the prevention of surgical site infection. November 2016. www.who.int/gpsc/ssi‐prevention‐guidelines/en/ (accessed 20 January 2017).
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

