Lhx1/5 control dendritogenesis and spine morphogenesis of Purkinje cells via regulation of Espin
- PMID: 28516904
- PMCID: PMC5454373
- DOI: 10.1038/ncomms15079
Lhx1/5 control dendritogenesis and spine morphogenesis of Purkinje cells via regulation of Espin
Abstract
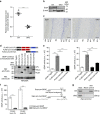
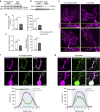
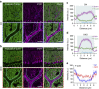
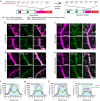
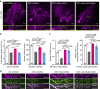
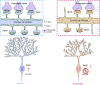
In the cerebellar cortex, Purkinje cells (PCs) receive signals from different inputs through their extensively branched dendrites and serve as an integration centre. Defects in the dendritic development of PCs thus disrupt cerebellar circuitry and cause ataxia. Here we report that specific inactivation of both Lhx1 and Lhx5 in postnatal PCs results in ataxic mutant mice with abnormal dendritic development. The PCs in the mutants have reduced expression of Espin, an F-actin cytoskeleton regulator. We show that Espin expression is transcriptionally activated by Lhx1/5. Downregulation of Espin leads to F-actin mislocalization, thereby impairing dendritogenesis and dendritic spine maturation in the PCs. The mutant PCs therefore fail to form proper synapses and show aberrant electrophysiological properties. By overexpressing Espin, we can successfully rescue the defects in the mutant PCs. Our findings suggest that Lhx1/5, through regulating Espin expression, control dendritogenesis and spine morphogenesis in postnatal PCs.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




Similar articles
-
Novel espin actin-bundling proteins are localized to Purkinje cell dendritic spines and bind the Src homology 3 adapter protein insulin receptor substrate p53.J Neurosci. 2003 Feb 15;23(4):1310-9. doi: 10.1523/JNEUROSCI.23-04-01310.2003. J Neurosci. 2003. PMID: 12598619 Free PMC article.
-
The RhoGEF DOCK10 is essential for dendritic spine morphogenesis.Mol Biol Cell. 2015 Jun 1;26(11):2112-27. doi: 10.1091/mbc.E14-08-1310. Epub 2015 Apr 7. Mol Biol Cell. 2015. PMID: 25851601 Free PMC article.
-
RORα Regulates Multiple Aspects of Dendrite Development in Cerebellar Purkinje Cells In Vivo.J Neurosci. 2015 Sep 9;35(36):12518-34. doi: 10.1523/JNEUROSCI.0075-15.2015. J Neurosci. 2015. PMID: 26354918 Free PMC article.
-
New waves in dendritic spine actin cytoskeleton: From branches and bundles to rings, from actin binding proteins to post-translational modifications.Mol Cell Neurosci. 2017 Oct;84:77-84. doi: 10.1016/j.mcn.2017.05.002. Epub 2017 May 4. Mol Cell Neurosci. 2017. PMID: 28479292 Review.
-
Aging of cerebellar Purkinje cells.Cell Tissue Res. 2010 Sep;341(3):341-7. doi: 10.1007/s00441-010-1016-2. Epub 2010 Jul 23. Cell Tissue Res. 2010. PMID: 20652318 Review.
Cited by
-
Disrupted development of sensory systems and the cerebellum in a zebrafish ebf3a mutant.G3 (Bethesda). 2025 Jul 9;15(7):jkaf115. doi: 10.1093/g3journal/jkaf115. G3 (Bethesda). 2025. PMID: 40406967 Free PMC article.
-
The islet-expressed Lhx1 transcription factor interacts with Islet-1 and contributes to glucose homeostasis.Am J Physiol Endocrinol Metab. 2019 Mar 1;316(3):E397-E409. doi: 10.1152/ajpendo.00235.2018. Epub 2019 Jan 8. Am J Physiol Endocrinol Metab. 2019. PMID: 30620636 Free PMC article.
-
Deep Brain Stimulation of the Interposed Nucleus Reverses Motor Deficits and Stimulates Production of Anti-inflammatory Cytokines in Ataxia Mice.Mol Neurobiol. 2022 Jul;59(7):4578-4592. doi: 10.1007/s12035-022-02872-w. Epub 2022 May 17. Mol Neurobiol. 2022. PMID: 35581519
-
Transcriptome programs involved in the development and structure of the cerebellum.Cell Mol Life Sci. 2021 Oct;78(19-20):6431-6451. doi: 10.1007/s00018-021-03911-w. Epub 2021 Aug 18. Cell Mol Life Sci. 2021. PMID: 34406416 Free PMC article. Review.
-
Real-time field-programmable gate array-based closed-loop deep brain stimulation platform targeting cerebellar circuitry rescues motor deficits in a mouse model of cerebellar ataxia.CNS Neurosci Ther. 2024 Mar;30(3):e14638. doi: 10.1111/cns.14638. CNS Neurosci Ther. 2024. PMID: 38488445 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

