Electrostatic melting in a single-molecule field-effect transistor with applications in genomic identification
- PMID: 28516911
- PMCID: PMC5454367
- DOI: 10.1038/ncomms15450
Electrostatic melting in a single-molecule field-effect transistor with applications in genomic identification
Abstract
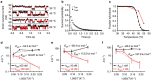
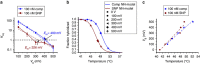
The study of biomolecular interactions at the single-molecule level holds great potential for both basic science and biotechnology applications. Single-molecule studies often rely on fluorescence-based reporting, with signal levels limited by photon emission from single optical reporters. The point-functionalized carbon nanotube transistor, known as the single-molecule field-effect transistor, is a bioelectronics alternative based on intrinsic molecular charge that offers significantly higher signal levels for detection. Such devices are effective for characterizing DNA hybridization kinetics and thermodynamics and enabling emerging applications in genomic identification. In this work, we show that hybridization kinetics can be directly controlled by electrostatic bias applied between the device and the surrounding electrolyte. We perform the first single-molecule experiments demonstrating the use of electrostatics to control molecular binding. Using bias as a proxy for temperature, we demonstrate the feasibility of detecting various concentrations of 20-nt target sequences from the Ebolavirus nucleoprotein gene in a constant-temperature environment.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
Label-free single-molecule detection of DNA-hybridization kinetics with a carbon nanotube field-effect transistor.Nat Nanotechnol. 2011 Feb;6(2):126-32. doi: 10.1038/nnano.2010.275. Epub 2011 Jan 23. Nat Nanotechnol. 2011. PMID: 21258331 Free PMC article.
-
Electrically Controllable Single-Point Covalent Functionalization of Spin-Cast Carbon-Nanotube Field-Effect Transistor Arrays.ACS Nano. 2018 Oct 23;12(10):9922-9930. doi: 10.1021/acsnano.8b03073. Epub 2018 Oct 3. ACS Nano. 2018. PMID: 30260623 Free PMC article.
-
Carbon nanotube transistors for biosensing applications.Anal Bioanal Chem. 2006 Jan;384(2):322-35. doi: 10.1007/s00216-005-3400-4. Epub 2005 Aug 30. Anal Bioanal Chem. 2006. PMID: 16132132 Review.
-
Label-free DNA biosensors based on functionalized carbon nanotube field effect transistors.Nano Lett. 2009 Feb;9(2):530-6. doi: 10.1021/nl8025604. Nano Lett. 2009. PMID: 19125575
-
Strategy for carrier control in carbon nanotube transistors.ChemSusChem. 2011 Jul 18;4(7):890-904. doi: 10.1002/cssc.201000412. Epub 2011 May 6. ChemSusChem. 2011. PMID: 21557492 Review.
Cited by
-
Ion-Selective Carbon Nanotube Field-Effect Transistors for Monitoring Drug Effects on Nicotinic Acetylcholine Receptor Activation in Live Cells.Sensors (Basel). 2020 Jun 30;20(13):3680. doi: 10.3390/s20133680. Sensors (Basel). 2020. PMID: 32630098 Free PMC article.
-
Molecular electronics sensors on a scalable semiconductor chip: A platform for single-molecule measurement of binding kinetics and enzyme activity.Proc Natl Acad Sci U S A. 2022 Feb 1;119(5):e2112812119. doi: 10.1073/pnas.2112812119. Proc Natl Acad Sci U S A. 2022. PMID: 35074874 Free PMC article.
-
RNA adapts its flexibility to efficiently fold and resist unfolding.bioRxiv [Preprint]. 2024 Nov 5:2024.05.27.595525. doi: 10.1101/2024.05.27.595525. bioRxiv. 2024. Update in: Nucleic Acids Res. 2025 Jul 19;53(14):gkaf681. doi: 10.1093/nar/gkaf681. PMID: 38853856 Free PMC article. Updated. Preprint.
-
RNA adapts its flexibility to efficiently fold and resist unfolding.Nucleic Acids Res. 2025 Jul 19;53(14):gkaf681. doi: 10.1093/nar/gkaf681. Nucleic Acids Res. 2025. PMID: 40737089 Free PMC article.
-
Advancements in Single-Molecule Fluorescence Detection Techniques and Their Expansive Applications in Drug Discovery and Neuroscience.Biosensors (Basel). 2025 Apr 30;15(5):283. doi: 10.3390/bios15050283. Biosensors (Basel). 2025. PMID: 40422023 Free PMC article. Review.
References
-
- Williams M. C. & Rouzina I. Force spectroscopy of single DNA and RNA molecules. Curr. Opin. Struc. Biol. 12, 330–336 (2002). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

