High conductance values in π-folded molecular junctions
- PMID: 28516950
- PMCID: PMC5454372
- DOI: 10.1038/ncomms15195
High conductance values in π-folded molecular junctions
Abstract
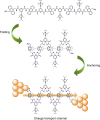
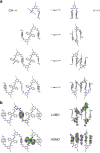
Folding processes play a crucial role in the development of function in biomacromolecules. Recreating this feature on synthetic systems would not only allow understanding and reproducing biological functions but also developing new functions. This has inspired the development of conformationally ordered synthetic oligomers known as foldamers. Herein, a new family of foldamers, consisting of an increasing number of anthracene units that adopt a folded sigmoidal conformation by a combination of intramolecular hydrogen bonds and aromatic interactions, is reported. Such folding process opens up an efficient through-space charge transport channel across the interacting anthracene moieties. In fact, single-molecule conductance measurements carried out on this series of foldamers, using the scanning tunnelling microscopy-based break-junction technique, reveal exceptionally high conductance values in the order of 10-1 G0 and a low length decay constant of 0.02 Å-1 that exceed the values observed in molecular junctions that make use of through-space charge transport pathways.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Gellman S. H. Foldamers: a manifesto. Acc. Chem. Res. 31, 173–180 (1998).
-
- Hill D. J., Mio M. J., Prince R. B., Hughes T. S. & Moore J. S. A field guide to foldamers. Chem. Rev. 101, 3893–4012 (2001). - PubMed
-
- eds Hecht S., Huc I. Foldamers: Structure, Properties, and Applications Wiley (2007).
-
- Guichard G. & Huc I. Synthetic foldamers. Chem. Commun. 47, 5933–5941 (2011). - PubMed
-
- Zhang D. W., Zhao X., Hou J. L. & Li Z. T. Aromatic amide foldamers: structures, properties, and functions. Chem. Rev. 112, 5271–5316 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

