Inhibition of the Continuum of Radiation-Induced Normal Tissue Injury by a Redox-Active Mn Porphyrin
- PMID: 28517962
- PMCID: PMC5559715
- DOI: 10.1667/RR14757.1.S1
Inhibition of the Continuum of Radiation-Induced Normal Tissue Injury by a Redox-Active Mn Porphyrin
Abstract
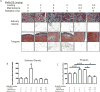
Normal tissue damage after head and neck radiotherapy involves a continuum of pathologic events to the mucosa, tongue and salivary glands. We examined the radioprotective effects of MnBuOE, a redox-active manganese porphyrin, at three stages of normal tissue damage: immediate (leukocyte endothelial cell [L/E] interactions), early (mucositis) and late (xerostomia and fibrosis) after treatment. In this study, mice received 0 or 9 Gy irradiation to the oral cavity and salivary glands ± MnBuOE treatment. Changes in leukocyte-endothelial cell interactions were measured 24 h postirradiation. At 11 days postirradiation, mucositis was assessed with a cathepsin-sensitive near-infrared optical probe. Stimulated saliva production was quantified at 11 weeks postirradiation. Finally, histological analyses were conducted to assess the extent of long-term effects in salivary glands at 12 weeks postirradiation. MnBuOE reduced oral mucositis, xerostomia and salivary gland fibrosis after irradiation. Additionally, although we have previously shown that MnBuOE does not interfere with tumor control at high doses when administered with radiation alone, most head and neck cancer patients will be treated with the combinations of radiotherapy and cisplatin. Therefore, we also evaluated whether MnBuOE would protect tumors against radiation and cisplatin using tumor growth delay as an endpoint. Using a range of radiation doses, we saw no evidence that MnBuOE protected tumors from radiation and cisplatin. We conclude that MnBuOE radioprotects normal tissue at both early and late time points, without compromising anti-tumor effects of radiation and cisplatin.
Figures

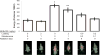
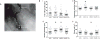
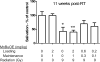


Similar articles
-
Novel Manganese-Porphyrin Superoxide Dismutase-Mimetic Widens the Therapeutic Margin in a Preclinical Head and Neck Cancer Model.Int J Radiat Oncol Biol Phys. 2015 Nov 15;93(4):892-900. doi: 10.1016/j.ijrobp.2015.07.2283. Epub 2015 Jul 29. Int J Radiat Oncol Biol Phys. 2015. PMID: 26530759 Free PMC article.
-
Mitigation of Radiation-Induced Epithelial Damage by the TLR5 Agonist Entolimod in a Mouse Model of Fractionated Head and Neck Irradiation.Radiat Res. 2017 May;187(5):570-580. doi: 10.1667/RR14514.1. Epub 2017 Mar 21. Radiat Res. 2017. PMID: 28323577 Free PMC article.
-
CNS bioavailability and radiation protection of normal hippocampal neurogenesis by a lipophilic Mn porphyrin-based superoxide dismutase mimic, MnTnBuOE-2-PyP5.Redox Biol. 2017 Aug;12:864-871. doi: 10.1016/j.redox.2017.04.027. Epub 2017 Apr 22. Redox Biol. 2017. PMID: 28454069 Free PMC article.
-
Salivary gland sparing in the treatment of head and neck cancer.Expert Rev Anticancer Ther. 2011 Sep;11(9):1437-48. doi: 10.1586/era.11.101. Expert Rev Anticancer Ther. 2011. PMID: 21929317 Review.
-
Current ideas to reduce or salvage radiation damage to salivary glands.Oral Dis. 2015 Jan;21(1):e1-10. doi: 10.1111/odi.12222. Epub 2014 Feb 28. Oral Dis. 2015. PMID: 24581290 Review.
Cited by
-
Therapeutic Potential of BMX-001 for Preventing Chemotherapy-Induced Peripheral Neuropathic Pain.Pharmaceuticals (Basel). 2025 Aug 5;18(8):1159. doi: 10.3390/ph18081159. Pharmaceuticals (Basel). 2025. PMID: 40872550 Free PMC article.
-
H2O2-Driven Anticancer Activity of Mn Porphyrins and the Underlying Molecular Pathways.Oxid Med Cell Longev. 2021 Mar 15;2021:6653790. doi: 10.1155/2021/6653790. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33815656 Free PMC article. Review.
-
Oxidative Stress and Chemoradiation-Induced Oral Mucositis: A Scoping Review of In Vitro, In Vivo and Clinical Studies.Int J Mol Sci. 2022 Apr 27;23(9):4863. doi: 10.3390/ijms23094863. Int J Mol Sci. 2022. PMID: 35563254 Free PMC article.
-
Will Nigella sativa oil protect parotid glands of rats against cranium gamma irradiation? Histological and immunohistochemical evaluation.BMC Complement Med Ther. 2024 Mar 6;24(1):111. doi: 10.1186/s12906-024-04410-8. BMC Complement Med Ther. 2024. PMID: 38448931 Free PMC article.
-
Application of a Novel Murine Ear Vein Model to Evaluate the Effects of a Vascular Radioprotectant on Radiation-Induced Vascular Permeability and Leukocyte Adhesion.Radiat Res. 2018 Jul;190(1):12-21. doi: 10.1667/RR14896.1. Epub 2018 Apr 19. Radiat Res. 2018. PMID: 29671690 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. Cancer J Clin. 2016;66:7–30. - PubMed
-
- Vissink A, Jansma J, Spijkervet FKL, Burlage FR, Coppes RP. Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14:199–212. - PubMed
-
- Giro C, Berger B, Boelke E, Ciernik IF, Duprez F, Locati L, et al. High rate of severe radiation dermatitis during radiation therapy with concurrent cetuximab in head and neck cancer: Results of a survey in EORTC institutes. Radiother Oncol. 2009;90:166–71. - PubMed
-
- Elting LS, Cooksley CD, Chambers MS, Garden AS. Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int J Radiat Oncol Biol Phys. 2007;68:1110–20. - PubMed
-
- Brizel DM, Wasserman TH, Henke M, Strnad V, Rudat V, Monnier A, et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol. 2000;18:3339–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

