The Toll-like receptor 9 signalling pathway regulates MR1-mediated bacterial antigen presentation in B cells
- PMID: 28518215
- PMCID: PMC5588774
- DOI: 10.1111/imm.12759
The Toll-like receptor 9 signalling pathway regulates MR1-mediated bacterial antigen presentation in B cells
Abstract
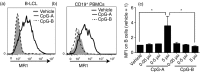
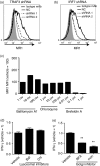
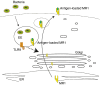
Mucosal-associated invariant T (MAIT) cells are conserved T cells that express a semi-invariant T-cell receptor (Vα7.2 in humans and Vα19 in mice). The development of MAIT cells requires the antigen-presenting MHC-related protein 1 (MR1), as well as commensal bacteria. The mechanisms that regulate the functional expression of MR1 molecules and their loading with bacterial antigen in antigen-presenting cells are largely unknown. We have found that treating B cells with the Toll-like receptor 9 (TLR9) agonist CpG increases MR1 surface expression. Interestingly, activation of TLR9 by CpG-A (but not CpG-B) enhances MR1 surface expression. This is limited to B cells and not other types of cells such as monocytes, T or natural killer cells. Knocking-down TLR9 expression by short hairpin RNA reduces MR1 surface expression and MR1-mediated bacterial antigen presentation. CpG-A triggers early endosomal TLR9 activation, whereas CpG-B is responsible for late endosomal/lysosomal activation of TLR9. Consistently, blocking endoplasmic reticulum to Golgi protein transport, rather than lysosomal acidification, suppressed MR1 antigen presentation. Overall, our results indicate that early endosomal TLR9 activation is important for MR1-mediated bacterial antigen presentation.
Keywords: B cells; Toll-like receptors; antigen presentation/processing; bacterial; signal transduction.
© 2017 John Wiley & Sons Ltd.
Figures




Similar articles
-
MR1 antigen presentation to MAIT cells: new ligands, diverse pathways?Curr Opin Immunol. 2018 Jun;52:108-113. doi: 10.1016/j.coi.2018.04.022. Epub 2018 May 10. Curr Opin Immunol. 2018. PMID: 29754112 Review.
-
TLR signaling in human antigen-presenting cells regulates MR1-dependent activation of MAIT cells.Eur J Immunol. 2016 Jul;46(7):1600-14. doi: 10.1002/eji.201545969. Epub 2016 May 30. Eur J Immunol. 2016. PMID: 27105778 Free PMC article.
-
The intracellular pathway for the presentation of vitamin B-related antigens by the antigen-presenting molecule MR1.Nat Immunol. 2016 May;17(5):531-7. doi: 10.1038/ni.3416. Epub 2016 Apr 4. Nat Immunol. 2016. PMID: 27043408
-
MAIT, MR1, microbes and riboflavin: a paradigm for the co-evolution of invariant TCRs and restricting MHCI-like molecules?Immunogenetics. 2016 Aug;68(8):537-48. doi: 10.1007/s00251-016-0927-9. Epub 2016 Jul 8. Immunogenetics. 2016. PMID: 27393664 Review.
-
Endosomal MR1 Trafficking Plays a Key Role in Presentation of Mycobacterium tuberculosis Ligands to MAIT Cells.PLoS Pathog. 2016 Mar 31;12(3):e1005524. doi: 10.1371/journal.ppat.1005524. eCollection 2016 Mar. PLoS Pathog. 2016. PMID: 27031111 Free PMC article.
Cited by
-
Implications of siRNA Therapy in Bone Health: Silencing Communicates.Biomedicines. 2024 Jan 1;12(1):90. doi: 10.3390/biomedicines12010090. Biomedicines. 2024. PMID: 38255196 Free PMC article. Review.
-
Role of innate T cells in necrotizing enterocolitis.Front Immunol. 2024 Feb 8;15:1357483. doi: 10.3389/fimmu.2024.1357483. eCollection 2024. Front Immunol. 2024. PMID: 38390341 Free PMC article. Review.
-
Mucosal-Associated Invariant T Cell Interactions with Commensal and Pathogenic Bacteria: Potential Role in Antimicrobial Immunity in the Child.Front Immunol. 2017 Dec 15;8:1837. doi: 10.3389/fimmu.2017.01837. eCollection 2017. Front Immunol. 2017. PMID: 29326714 Free PMC article. Review.
-
Sophisticated specificity in the innate immune response.Immunology. 2019 Oct;158(2):61-62. doi: 10.1111/imm.13112. Immunology. 2019. PMID: 31515802 Free PMC article.
-
MR1 antigen presentation to MAIT cells and other MR1-restricted T cells.Nat Rev Immunol. 2024 Mar;24(3):178-192. doi: 10.1038/s41577-023-00934-1. Epub 2023 Sep 29. Nat Rev Immunol. 2024. PMID: 37773272 Free PMC article. Review.
References
-
- Le Bourhis L, Guerri L, Dusseaux M, Martin E, Soudais C, Lantz O. Mucosal‐associated invariant T cells: unconventional development and function. Trends Immunol 2011; 32:212–8. - PubMed
-
- Le Bourhis L, Martin E, Peguillet I, Guihot A, Froux N, Core M et al Antimicrobial activity of mucosal‐associated invariant T cells. Nat Immunol 2010; 11:701–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

