Impact of the timing of hepatitis B virus identification and anti-hepatitis B virus therapy initiation on the risk of adverse liver outcomes for patients receiving cancer therapy
- PMID: 28518219
- PMCID: PMC5568919
- DOI: 10.1002/cncr.30729
Impact of the timing of hepatitis B virus identification and anti-hepatitis B virus therapy initiation on the risk of adverse liver outcomes for patients receiving cancer therapy
Abstract
Background: Data on the incidence of adverse liver outcomes are limited for cancer patients with chronic (hepatitis B surface antigen [HBsAg]-positive/hepatitis B core antibody [anti-HBc]-positive) or past (HBsAg-negative/anti-HBc-positive) hepatitis B virus (HBV) after chemotherapy. This study was aimed at determining the impact of test timing and anti-HBV therapy on adverse liver outcomes in these patients.
Methods: Patients with solid or hematologic malignancies who received chemotherapy between 2004 and 2011 were retrospectively studied. HBV testing and anti-HBV therapy were defined as early at the initiation of cancer therapy and as late after initiation. Outcomes included hepatitis flares, hepatic impairment, liver failure, and death. Time-to-event analysis was used to determine incidence, and multivariate hazard models were used to determine predictors of outcomes.
Results: There were 18,688 study patients (80.4% with solid tumors). The prevalence of chronic HBV was 1.1% (52 of 4905), and the prevalence of past HBV was 7.1% (350 of 4905). Among patients with solid tumors, late identification of chronic HBV was associated with a higher risk of hepatitis flare (hazard ratio [HR], 4.02; 95% confidence interval [CI], 1.26-12.86), hepatic impairment (HR, 8.48; 95% CI, 1.86-38.66), liver failure (HR, 9.38; 95% CI, 1.50-58.86), and death (HR, 3.90; 95% CI, 1.19-12.83) in comparison with early identification. Among patients with hematologic malignancies and chronic HBV, the risk of death was 7.8 (95% CI, 1.73-35.27) times higher for persons with late initiation of anti-HBV therapy versus early initiation. Patients with late identification of chronic HBV had late or no anti-HBV therapy. Chronic HBV predicted liver failure in patients with solid or hematologic malignancies, whereas male sex and late identification were predictors for patients with solid tumors.
Conclusions: Early identification correlates with early anti-HBV therapy and reduces the risk of liver failure and death in chronic HBV patients receiving chemotherapy. Cancer 2017;123:3367-76. © 2017 American Cancer Society.
Keywords: antiviral therapy; cancer; hematologic malignancies; hepatitis B; hepatitis B reactivation; hepatitis B screening; hepatitis flare; liver failure.
© 2017 American Cancer Society.
Conflict of interest statement
Figures

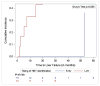
References
-
- World Health Organization. [Accessed April 16, 2015];Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. http://apps.who.int/iris/handle/10665/154590. - PubMed
-
- Baden LR, Swaminathan S, Angarone M, et al. Prevention and Treatment of Cancer-Related Infections, Version 2.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14(7):882–913. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

