Branched-chain amino acids for people with hepatic encephalopathy
- PMID: 28518283
- PMCID: PMC6481897
- DOI: 10.1002/14651858.CD001939.pub4
Branched-chain amino acids for people with hepatic encephalopathy
Update in
-
Branched-chain amino acids for people with cirrhosis and hepatic encephalopathy.Cochrane Database Syst Rev. 2026 Jan 16;1(1):CD001939. doi: 10.1002/14651858.CD001939.pub5. Cochrane Database Syst Rev. 2026. PMID: 41542879
Abstract
Background: Hepatic encephalopathy is a brain dysfunction with neurological and psychiatric changes associated with liver insufficiency or portal-systemic shunting. The severity ranges from minor symptoms to coma. A Cochrane systematic review including 11 randomised clinical trials on branched-chain amino acids (BCAA) versus control interventions has evaluated if BCAA may benefit people with hepatic encephalopathy.
Objectives: To evaluate the beneficial and harmful effects of BCAA versus any control intervention for people with hepatic encephalopathy.
Search methods: We identified trials through manual and electronic searches in The Cochrane Hepato-Biliary Group Controlled Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, Science Citation Index Expanded and Conference Proceedings Citation Index - Science, and LILACS (May 2017).
Selection criteria: We included randomised clinical trials, irrespective of the bias control, language, or publication status.
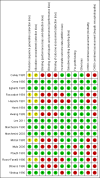
Data collection and analysis: The authors independently extracted data based on published reports and collected data from the primary investigators. We changed our primary outcomes in this update of the review to include mortality (all cause), hepatic encephalopathy (number of people without improved manifestations of hepatic encephalopathy), and adverse events. The analyses included random-effects and fixed-effect meta-analyses. We performed subgroup, sensitivity, regression, and trial sequential analyses to evaluate sources of heterogeneity (including intervention, and participant and trial characteristics), bias (using The Cochrane Hepato-Biliary Group method), small-study effects, and the robustness of the results after adjusting for sparse data and multiplicity. We graded the quality of the evidence using the GRADE approach.
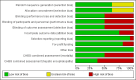
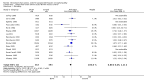
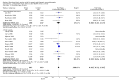
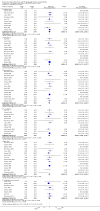
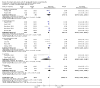
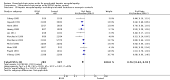
Main results: We found 16 randomised clinical trials including 827 participants with hepatic encephalopathy classed as overt (12 trials) or minimal (four trials). Eight trials assessed oral BCAA supplements and seven trials assessed intravenous BCAA. The control groups received placebo/no intervention (two trials), diets (10 trials), lactulose (two trials), or neomycin (two trials). In 15 trials, all participants had cirrhosis. We classed seven trials as low risk of bias and nine trials as high risk of bias (mainly due to lack of blinding or for-profit funding). In a random-effects meta-analysis of mortality, we found no difference between BCAA and controls (risk ratio (RR) 0.88, 95% confidence interval (CI) 0.69 to 1.11; 760 participants; 15 trials; moderate quality of evidence). We found no evidence of small-study effects. Sensitivity analyses of trials with a low risk of bias found no beneficial or detrimental effect of BCAA on mortality. Trial sequential analysis showed that the required information size was not reached, suggesting that additional evidence was needed. BCAA had a beneficial effect on hepatic encephalopathy (RR 0.73, 95% CI 0.61 to 0.88; 827 participants; 16 trials; high quality of evidence). We found no small-study effects and confirmed the beneficial effect of BCAA in a sensitivity analysis that only included trials with a low risk of bias (RR 0.71, 95% CI 0.52 to 0.96). The trial sequential analysis showed that firm evidence was reached. In a fixed-effect meta-analysis, we found that BCAA increased the risk of nausea and vomiting (RR 5.56; 2.93 to 10.55; moderate quality of evidence). We found no beneficial or detrimental effects of BCAA on nausea or vomiting in a random-effects meta-analysis or on quality of life or nutritional parameters. We did not identify predictors of the intervention effect in the subgroup, sensitivity, or meta-regression analyses. In sensitivity analyses that excluded trials with a lactulose or neomycin control, BCAA had a beneficial effect on hepatic encephalopathy (RR 0.76, 95% CI 0.63 to 0.92). Additional sensitivity analyses found no difference between BCAA and lactulose or neomycin (RR 0.66, 95% CI 0.34 to 1.30).
Authors' conclusions: In this updated review, we included five additional trials. The analyses showed that BCAA had a beneficial effect on hepatic encephalopathy. We found no effect on mortality, quality of life, or nutritional parameters, but we need additional trials to evaluate these outcomes. Likewise, we need additional randomised clinical trials to determine the effect of BCAA compared with interventions such as non-absorbable disaccharides, rifaximin, or other antibiotics.
Conflict of interest statement
None of the authors have any financial interests that may conflict with this review.
Lise Lotte Gluud had previously conducted Cochrane systematic reviews on the treatment of hepatic encephalopathy. As explained in the Cochrane Hepato‐Biliary Group module (Gluud 2017), this entails a risk of academic bias. Lise Lotte Gluud participated in scientific meetings in Denmark sponsored by Norgine. Giulio Marchesini, Iñigo Les, and Hendrik Vilstrup had previously conducted clinical trials on BCAA, which also entails a risk of academic bias. Niels Kristian Aagaard, Gitte Dam, and Mette Borre have no conflicts of interest.
Figures
















Update of
-
Branched-chain amino acids for people with hepatic encephalopathy.Cochrane Database Syst Rev. 2015 Sep 17;(9):CD001939. doi: 10.1002/14651858.CD001939.pub3. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2017 May 18;5:CD001939. doi: 10.1002/14651858.CD001939.pub4. PMID: 26377410 Updated.
Comment in
-
Branched-chain Amino Acids for People with Hepatic Encephalopathy.Am Fam Physician. 2020 Jan 1;101(1):Online. Am Fam Physician. 2020. PMID: 31894931 No abstract available.
References
References to studies included in this review
Calvey 1985 {published data only}
-
- Calvey H, Davis M, Williams R. Controlled trial of nutritional supplementation, with and without branched chain amino acid enrichment, in treatment of acute alcoholic hepatitis. Journal of Hepatology 1985;1:141‐51. - PubMed
-
- Calvey H, Davis M, Williams R. Prospective study of nasogastric feeding via East Grinstead or Viomedex tubes compared with oral dietary supplementation in patients with cirrhosis. Clinical Nutrition 1984;3:63‐6. - PubMed
-
- Williams R, Calvey H, Davis M. Controlled trial of nutritional supplementation in acute alcoholic hepatitis. In: Holm EKH editor(s). Metabolism and Nutrition in Liver Disease. Lancaster, UK: MTP Press, Ltd, 1985:361‐8.
Cerra 1985 {published and unpublished data}
-
- Cerra FB. Disease‐specific amino acid infusion (F080) in hepatic encephalopathy: a prospective, randomized, double‐blind, controlled trial [personal communication]. Correspondence with authors September 2002.
-
- Cerra FB, Cheung NK, Fischer JE, Kaplowitz N, Schiff E‐R, Dienstag JL, et al. Disease‐specific amino acid infusion (F080) in hepatic encephalopathy: a prospective, randomized, double‐blind, controlled trial. JPEN. Journal of Parenteral and Enteral Nutrition 1985;9:288‐95. - PubMed
-
- Cerra FB, Cheung NK, Fischer JE, Kaplowitz N, Schiff ER, Dienstag JL, et al. A multicenter trial of branched chain enriched amino acid infusion (F080) in hepatic encephalopathy (HE). Hepatology (Baltimore, Md.) 1982;2:699.
-
- Cerra FB, McMillen M, Angelico R, Cline B, Lyons J, Faulkenbach L, et al. Cirrhosis, encephalopathy, and improved results with metabolic support. Surgery 1983;94:612‐9. - PubMed
Egberts 1985 {published data only}
-
- Egberts EH, Hamster W, Schomeerus H, Jürgens P. Effect of branched chain amino acids on latent porto‐systemic‐encephalopathy (PSE). JPEN. Journal of Parenteral and Enteral Nutrition 1981;5:5.
-
- Egberts EH, Schomeerus H, Hamster W, Jürgens P. Effective treatment of latens porto‐systemic encephalopathy with oral branched chain amino acids. In: Capocaccia L, Fischer JE, Rossi‐Fanelli F editor(s). Hepatic Encephalopathy in Chronic Liver Failure. New York: Plenum Press, 1984:351‐7.
-
- Egberts EH, Schomerus H, Hamster W, Jurgens P. Branched chain amino acids in the treatment of latent portosystemic encephalopathy. A double‐blind placebo‐controlled crossover study. Gastroenterology 1985;88:887‐95. - PubMed
-
- Egberts EH, Schomerus H, Hamster W, Jürgens P. Branched‐chain amino acids in the treatment of latent porto‐systemic encephalopathy. A placebo‐controlled double‐blind cross‐over study [Verzweigtkettige Aminosäuren bei der behandlung der latenten portosystemischen enzephalopathie. Eine placebokontrollierte doppelblind‐cross‐over‐studie]. Zeitschrift für Ernährungswissenschaft 1986;25:9‐28. - PubMed
-
- Hamster W, Egberts EH, Hamster H. Treatment with branched‐chain amino acids and effect on psycho‐physical capacity functions in latent porto‐systemic encephalopathy [Behandlung mit verzweigtkettigen aminosäuren und ihre auswirkung auf psychophysische leistungsfunktionen bei latenter portosystemischer enzephalopathie]. Arzneimittelforschung 1982;32:901‐2.
Fiaccadori 1984 {published and unpublished data}
-
- Fiaccadori F. Branched chain amino acid enriched solutions in the treatment of hepatic encephalopathy: a controlled trial. Italian Journal of Gastroenterology (additional information from author November 2012).
-
- Fiaccadori F, Ghinelli F, Pedretti G. Branched chain amino acid enriched solutions in the treatment of hepatic encephalopathy: a controlled trial. Italian Journal of Gastroenterology 1985;17:5‐10.
-
- Fiaccadori F, Ghinelli F, Pedretti G, Mancia D. Mental state course and biochemical findings in HE treated by BCAA‐enriched mixtures. In: Holm E, Kasper H editor(s). Metabolism and Nutrition in Liver Disease Freiburg 1984: Proceedings of the 41st Falk Symposium. Lancaster: MTP Press, 1985:281‐5.
-
- Fiaccadori F, Ghinelli F, Pedretti G, Pelosi G, Sacchini D, Zeneroli ML, et al. Branched chain amino acid enriched solutions in the treatment of hepatic encephalopathy: a controlled trial. In: Capocaccia L, Fischer JE, Rossi‐Fanelli F editor(s). Hepatic Encephalopathy in Chronic Liver Failure. New York: Plenum Press, 1984:323‐33.
-
- Fiaccadori F, Ghinelli F, Pelosi G, Sacchini D, Vaona G, Zeneroli ML, et al. Selective amino acid solutions in hepatic encephalopathy treatment (a preliminary report). La Ricerca in Clinica e in Laboratorio 1980;10:411‐22. [MEDLINE: ] - PubMed
Hayashi 1991 {published data only}
-
- Hayashi S, Aoyagi Y, Fujiwara K, Oka H, Oda T. A randomized controlled trial of branched‐chain amino acid (BCAA)‐enriched elemental diet (ED‐H) for hepatic encephalopathy. Journal of Gastroenterology and Hepatology 1991;6:191.
Horst 1984 {published data only}
-
- Horst D, Grace N, Conn HO, Schiff E, Schenker S, Viteri A, et al. A double‐blind randomized comparison of dietary protein and an oral branched chain amino acid (BCAA) solution in cirrhotic patients with chronic portal‐systemic encephalopathy (PSE) [IASL abstract]. Hepatology (Baltimore, Md.) 1982;2:184. - PubMed
-
- Horst D, Grace ND, Conn HO, Schiff E, Schenker S, Viteri A, et al. Comparison of dietary protein with an oral, branched chain‐enriched amino acid supplement in chronic portal‐systemic encephalopathy: a randomized controlled trial. Hepatology (Baltimore, Md.) 1984;4:279‐87. - PubMed
Hwang 1988 {published data only}
-
- Hwang SJ, Chan CY, Wu JC, Lee SD, Huan YS, Tsai YT, et al. A randomized controlled trial for the evaluation of the efficacy of branched chain amino acid‐enriched amino acid solution in the treatment of patients with hepatic encephalopathy. Chinese Journal of Gastroenterology 1988;5:185‐92.
Les 2011 {published data only}
-
- Les I, Doval E, Garcia‐Martinez R, Planas M, Cardenas G, Gomez P, et al. Effects of branched‐chain amino acids supplementation in patients with cirrhosis and a previous episode of hepatic encephalopathy: a randomized study. American Journal of Gastroenterology 2011;106:1081‐8. - PubMed
Marchesini 1990 {published data only}
-
- Bianchi GP, Marchesini G, Zoli M, Abbiati R, Ferrario E, Fabbri A, et al. Oral BCAA supplementation in cirrhosis with chronic encephalopathy: effects on prolactin and estradiol levels. Hepato‐gastroenterology 1992;39:443‐6. - PubMed
-
- Marchesini G, Bianchi G, Merli M, Amodio P, Panella C, Loguercio C, et al. Nutritional supplementation with branched‐chain amino acids in advanced cirrhosis: a double‐blind, randomized trial. Gastroenterology 2003;124:1792‐801. - PubMed
-
- Marchesini G, Dioguardi FS, Bianchi GP, Zoli M, Bellati G, Roffi L, et al. Long‐term oral branched‐chain amino acid treatment in chronic hepatic encephalopathy. A randomized double‐blind casein controlled trial. The Italian Multicenter Study Group. Journal of Hepatology 1990;11:92‐101. - PubMed
Marchesini 2003 {published and unpublished data}
-
- Marchesini G, Bianchi G, Merli M, Amodio P, Panella C, Loguercio C, et al. Nutritional supplementation with branched‐chain amino acids in advanced cirrhosis: a double‐blind, randomized trial. Gastroenterology 2003;124:1980‐2. - PubMed
Michel 1985 {published data only}
-
- Michel H, Bories P, Aubin JP, Pomier‐Layrargues G, Bauret P, Bellet‐Herman H. Treatment of acute hepatic encephalopathy in cirrhotics with a branched‐chain amino acids enriched versus a conventional amino acids mixture. A controlled study of 70 patients. Liver 1985;5:282‐9. - PubMed
-
- Michel H, Pomier‐Layrargues G, Aubin JP, Bories P, Mirouze D, Bellet‐Herman H. Treatment of hepatic encephalopathy by infusion of a modified amino acid solution: results of a controlled study in 47 cirrhotic patients. In: Capocaccia L, Fischer JE, Rossi‐Fanelli F editor(s). Hepatic Encephalopathy in Chronic Liver Failure. New York: Plenum Press, 1984:301‐10.
-
- Michel H, Pomier‐Layrargues G, Duhamel O, Lacombe B, Cuilleret G, Bellet‐Hermann H. Intravenous infusion of ordinary and modified amino‐acid solutions in the management of hepatic encephalopathy (controlled study, 30 patients). Gastroenterology 1980;79:1038.
-
- Pomier‐Layrargues G, Duhamel O, Lacombe B, Cuilleret G, Bellet H, Michel H. Intravenous infusion of ordinary and modified amino‐acid solutions in the management of hepatic encephalopathy. Liver 1981;1:140.
Muto 2005 {published and unpublished data}
-
- Horie‐Takashi I. Effects of oral branched‐chain amino acid granules on event‐free survival in patients with liver cirrhosis. Clinical Gastroenterology and Hepatology 2005; Vol. 3:705‐13 (additional information and data received November 2011). - PubMed
-
- Muto Y, Sato S, Watanabe A, Moriwaki H, Suzuki K, Kato A, et al. Effects of oral branched‐chain amino acid granules on event‐free survival in patients with liver cirrhosis. Clinical Gastroenterology and Hepatology 2005;3:705‐13. - PubMed
-
- Sato S, Watanabe A, Muto Y, Suzuki K, Kato A, Moriwaki H, et al. Clinical comparison of branched‐chain amino acid (l‐Leucine, l‐Isoleucine, l‐Valine) granules and oral nutrition for hepatic insufficiency in patients with decompensated liver cirrhosis (LIV‐EN study). Hepatology Research 2005;31:232‐40. - PubMed
Plauth 1993 {published data only}
-
- Plauth M, Egberts E‐H, Hamster W, Török M, Müller P, Brand O, et al. Long‐term treatment with branched‐chain amino acids (BCA) improves the portosystemic encephalopathy (PSE) in ambulant patients. Results of a double blind, placebo controlled crossover study. Klinische Wochenschrift 1992;69:126.
-
- Plauth M, Egberts EH, Hamster W, Török M, Müller PH, Brand O, et al. Long‐term treatment of latent portosystemic encephalopathy with branched‐chain amino acids. A double‐blind placebo‐crossover study. Journal of Hepatology 1993;17:308‐14. - PubMed
Rossi‐Fanelli 1986 {published data only}
-
- Riggio O, Cangiano C, Cascino A, Merli M, Stortoni M, Rossi‐Fanelli F, et al. Long term dietary supplement with branched chain amino acids: a new approach in the prevention of hepatic encephalopathy: results of a controlled study in cirrhotics with porto‐caval anastomosis. In: Associazione Italiana per Lo Studio del Fegato. Congress, editor(s). Hepatic Encephalopathy in Chronic Liver Failure. New York: Plenum Press, 1984:183‐92.
-
- Rossi‐Fanelli F, Cangiano C, Cascino A, Merli M, Riggio O, Stortoni M, et al. Branched‐chain amino acids in the treatment of severe hepatic encephalopathy. In: Capocaccia L, Fischer JE, Rossi‐Fanelli F editor(s). Hepatic Encephalopathy in Chronic Liver Failure. New York: Plenum Press, 1984:335‐44.
-
- Rossi‐Fanelli F, Riggio O, Cangiano C, Cascino A, Conciliis D, Merli M, et al. Branched‐chain amino acids vs lactulose in the treatment of hepatic coma: a controlled study. Digestive Diseases and Sciences 1982;27:929‐35. - PubMed
Strauss 1986 {published and unpublished data}
-
- Strauss E, Cartapatti Da Silva E, Lacet CM, Capacci MLL, Bernardini AP. Treatment of hepatic encephalopathy: a randomized clinical trial comparing a branched chain enriched amino acid solution to oral neomycin. Nutritional Support Services 1986;6:18‐21.
-
- Strauss E, Santos WR, Silva EC, Lacet CM, Capacci LL, Bernardini AP. A randomized controlled clinical trial for the evaluation of the efficacy of an enriched branched‐chain amino‐acid solution compared to neomycin in hepatic encephalopathy. Hepatology (Baltimore, Md.) 1983;3:862.
Vilstrup 1990 {published data only}
-
- Gluud C, Dejgaard A, Hardt F, Kristensen M, Køhler O, Melgaard B, et al. Preliminary treatment results with balanced amino acid infusion to patients with hepatic encephalopathy. Scandinavian Journal of Gastroenterology 1983;18:19.
-
- Vilstrup H, Gluud C, Hardt F, Kristensen M, Køhler O, Melgaard B, et al. Branched chain enriched amino acid versus glucose treatment of hepatic encephalopathy. A double‐blind study of 65 patients with cirrhosis. Hepatology (Baltimore, Md.) 1990;10:291‐6. - PubMed
-
- Vilstrup H, Gluud C, Hardt F, Kristensen M, Melgaard B, Køhler O, et al. Branched chain enriched amino acid nutrition does not change the outcome of hepatic coma in patients with cirrhosis of the liver. Journal of Hepatology 1985;1:S347. - PubMed
References to studies excluded from this review
Eriksson 1982 {published data only}
Hayashi 2007 {published data only}
-
- Hayashi M, Ikezawa K, Ono A, Okabayashi S, Hayashi Y, Shimizu S, et al. Evaluation of the effects of combination therapy with branched‐chain amino acid and zinc supplements on nitrogen metabolism in liver cirrhosis. Hepatology Research 2007;37:615‐19. - PubMed
Malaguarnera 2009 {published data only}
-
- Malaguarnera M, Risino C, Cammalleri L, Malaguarnera L, Astuto M, Vecchio I, et al. Branched chain amino acids supplemented with L‐acetylcarnitine versus BCAA treatment in hepatic coma: a randomized and controlled double blind study. European Journal of Gastroenterology & Hepatology 2009;21:762‐70. - PubMed
O'Keefe 1987 {published data only}
-
- O'Keefe SJ, Ogden J, Dicker J. Enteral and parenteral branched chain amino acid‐supplemented nutritional support in patients with encephalopathy due to alcoholic liver disease. JPEN. Journal of Parenteral and Enteral Nutrition 1987;11:447‐53. - PubMed
Wahren 1983 {published data only}
-
- Wahren J, Denis J, Desurmont P, Eriksson LS, Escoffier JM, Gauthier AP, et al. Is intravenous administration of branched chain amino acids effective in the treatment of hepatic encephalopathy? A multicenter study. Hepatology (Baltimore, Md.) 1983;3:475‐80. - PubMed
Walker 1982 {published data only}
-
- Walker S, Gotz R, Czygan P, Stiehl A, Lanzinger G, Sieg A, et al. Oral keto analogs of branched‐chain amino acids in hyperammonemia in patients with cirrhosis of the liver. A double‐blind crossover study. Digestion 1982;24:105‐11. - PubMed
References to ongoing studies
ACTRN12610001021066 {unpublished data only}
-
- The effects of supplementation with synbiotics, branched chain amino acids on levels of hepatic encephalopathy in patients with cirrhosis. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12610001021066 (accessed 10 May 2017).
Additional references
AASLD and EASL guideline 2014a
-
- Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology (Baltimore, Md.) 2014;60:715‐35. - PubMed
AASLD and EASL guideline 2014b
-
- American Association for the Study of Liver Diseases, European Association for the Study of the Liver. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. Journal of Hepatology 2014;61:642‐59. - PubMed
Amodio 2008
-
- Amodio P, Campagna F, Olianas S, Iannizzi P, Mapelli D, Penzo M, et al. Detection of minimal hepatic encephalopathy: normalization and optimization of the Psychometric Hepatic Encephalopathy Score. A neuropsychological and quantified EEG study. Journal of Hepatology 2008;49(3):346‐53. [PUBMED: 18602716] - PubMed
Amodio 2014
-
- Amodio P, Canesso F, Montagnese S. Dietary management of hepatic encephalopathy revisited. Current Opinion in Clinical Nutrition and Metabolic Care 2014;17(5):448‐52. [PUBMED: 25025262] - PubMed
Bajaj 2008
Bajaj 2010
-
- Bajaj JS. Review article: the modern management of hepatic encephalopathy. Alimentary Pharmacology & Therapeutics 2010;31(5):537‐47. [PUBMED: 20002027] - PubMed
Bajaj 2011
-
- Bajaj JS, Cordoba J, Mullen KD, Amodio P, Shawcross DL, Butterworth RF, et al. Review article: the design of clinical trials in hepatic encephalopathy ‐ an International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) consensus statement. Alimentary Pharmacology & Therapeutics 2011;33(7):739‐47. [PUBMED: 21306407] - PMC - PubMed
Bak 2006
-
- Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA‐glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. Journal of Neurochemistry 2006;98:641‐53. - PubMed
Blei 2001
-
- Blei AT, Córdoba J, Practice Parameters Committee of the American College of Gastroenterology. Hepatic encephalopathy. American Journal of Gastroenterology 2001;96:1986‐76. - PubMed
Córdoba 2004
-
- Córdoba J, López‐Hellín J, Planas M, Sabín P, Sanpedro F, Castro F, et al. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. Journal of Hepatology 2004;41:38‐43. - PubMed
Dam 2011
-
- Dam G, Keiding S, Munk OL, Ott P, Buhl M, Vilstrup H, et al. Branched‐chain amino acids increase arterial blood ammonia in spite of enhanced intrinsic muscle ammonia metabolism in patients with cirrhosis and healthy subjects. American Journal of Physiology. Gastrointestinal and Liver Physiology 2011;301(2):G269‐77. [PUBMED: 21636533] - PubMed
Egger 1997
Ferenci 2002
-
- Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy ‐ definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology (Baltimore, Md.) 2002;35(3):716‐21. [PUBMED: 11870389] - PubMed
Fichet 2009
-
- Fichet J, Mercier E, Genée O, Garot D, Legras A, Dequin PF, et al. Prognosis and 1‐year mortality of intensive care unit patients with severe hepatic encephalopathy. Journal of Critical Care 2009;24:364‐70. - PubMed
Garcia‐Martinez 2011
-
- García‐Martínez R, Córdoba J. Acute‐on‐chronic liver failure: the brain. Current Opinion in Critical Care 2011;17(2):177‐83. - PubMed
Gluud 1991
-
- Gluud C. Branched‐chain amino acids for hepatic encephalopathy?. Hepatology (Baltimore, Md.) 1991;13(4):812‐3. - PubMed
Gluud 2010
-
- Gluud LL, Langholz E, Krag A. Meta‐analysis: isosorbide‐mononitrate alone or with either beta‐blockers or endoscopic therapy for the management of oesophageal varices. Alimentary Pharmacology & Therapeutics 2010;32(7):859‐71. [PUBMED: 20839387] - PubMed
Gluud 2013a
-
- Gluud LL, Dam G, Borre M, Les I, Cordoba J, Marchesini G, et al. Oral branched‐chain amino acids have a beneficial effect on manifestations of hepatic encephalopathy in a systematic review with meta‐analyses of randomized controlled trials. Journal of Nutrition 2013;143:1263‐8. [PUBMED: 23739310] - PubMed
Gluud 2013b
-
- Gluud LL, Dam G, Borre M, Les I, Cordoba J, Marchesini G, et al. Lactulose, rifaximin or branched chain amino acids for hepatic encephalopathy: what is the evidence?. Metabolic Brain Disease 2013;28(2):221‐5. [PUBMED: 23275147] - PubMed
Gluud 2017
-
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2017, Issue 4. Art. No.: LIVER.
GRADEpro [Computer program]
-
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.2 for Windows. Grade Working Group 2004‐2007, 2008.
Guerit 2009
-
- Guerit JM, Amantini A, Fischer C, Kaplan PW, Mecarelli O, Schnitzler A, et al. Neurophysiological investigations of hepatic encephalopathy: ISHEN practice guidelines. Liver International 2009;29(6):789‐96. [PUBMED: 19638107] - PubMed
Guyatt 2008
Harbord 2006
-
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [PUBMED: 16345038] - PubMed
Higgins 2008
Higgins 2011a
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
Holecek 2010
-
- Holecek M. Three targets of branched‐chain amino acid supplementation in the treatment of liver disease. Nutrition (Burbank, Los Angeles County, Calif.) 2010;26(5):482‐90. [PUBMED: 20071143] - PubMed
Holecek 2011
-
- Holecek M, Kandar R, Sispera L, Kovarik M. Acute hyperammonemia activates branched‐chain amino acid catabolism and decreases their extracellular concentrations: different sensitivity of red and white muscle. Amino Acids 2011;40(2):575‐84. [PUBMED: 20614225] - PubMed
Holecek 2013
-
- Holecek M. Branched‐chain amino acids and ammonia metabolism in liver disease: therapeutic implications. Nutrition (Burbank, Los Angeles County, Calif.) 2013;29(10):1186‐91. [PUBMED: 23756281] - PubMed
Holecek 2014
ICH GCP 1997
-
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH Guidelines. Vol. 1, Philadelphia (PA): Barnett International/PAREXEL, 1997.
Kachaamy 2011
-
- Kachaamy T, Bajaj JS. Diet and cognition in chronic liver disease. Current Opinion Gastroenterology 2011;27:174‐9. - PubMed
Kawaguchi 2013
-
- Kawaguchi T, Taniguchi E, Sata M. Effects of oral branched‐chain amino acids on hepatic encephalopathy and outcome in patients with liver cirrhosis. Nutrition in Clinical Practice 2013;28(5):580‐8. [PUBMED: 23945292] - PubMed
Kircheis 2007
-
- Kircheis G, Fleig WE, Gortelmeyer R, Grafe S, Haussinger D. Assessment of low‐grade hepatic encephalopathy: a critical analysis. Journal of Hepatology 2007;47(5):642‐50. [PUBMED: 17869373] - PubMed
Koretz 2012
Lathyris 2007
-
- Lathyris DN, Trikalinos TA, Ioannidis JP. Evidence from crossover trials: empirical evaluation and comparison against parallel arm trials. International Journal of Epidemiology 2007;36(2):422‐30. [PUBMED: 17301102] - PubMed
MECIR 2014
-
- Methodological Expectations of Cochrane Intervention Reviews (MECIR). www.editorial‐unit.cochrane.org/mecir (accessed 25 August 2015).
Metcalfe 2014
-
- Metcalfe EL, Avenell A, Fraser A. Branched‐chain amino acid supplementation in adults with cirrhosis and porto‐systemic encephalopathy: systematic review. Clinical Nutrition (Edinburgh, Scotland) 2014;33(6):958‐65. [PUBMED: 24656171] - PubMed
Mullen 2007
-
- Mullen KD. Review of the final report of the 1998 Working Party on definition, nomenclature and diagnosis of hepatic encephalopathy. Alimentary Pharmacology & Therapeutics 2007;25(Suppl 1):11‐6. [PUBMED: 17295847] - PubMed
Naylor 1989
-
- Naylor CD, O'Rourke D, Detsky AS, Baker JP. Parenteral nutrition with branched‐amino acids in hepatic encephalopathy. A meta‐analysis. Gastroenterology 1989;97:1033‐42. - PubMed
Olde 2002
-
- Olde Damink SW, Jalan R, Redhead DN, Hayes PC, Deutz NE, Soeters PB. Interorgan ammonia and amino acid metabolism in metabolically stable patients with cirrhosis and a TIPSS. Hepatology (Baltimore, Md.) 2002;36(5):1163‐71. [PUBMED: 12395326] - PubMed
Rakoski 2012
Randolph 2009
-
- Randolph C, Hilsabeck R, Kato A, Kharbanda P, Li YY, Mapelli D, et al. Neuropsychological assessment of hepatic encephalopathy: ISHEN practice guidelines. Liver International 2009;29(5):629‐35. [PUBMED: 19302444] - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roman 2014
-
- Roman E, Torrades MT, Nadal MJ, Cardenas G, Nieto JC, Vidal S, et al. Randomized pilot study: effects of an exercise programme and leucine supplementation in patients with cirrhosis. Digestive Diseases and Sciences 2014;59(8):1966‐75. [PUBMED: 24599772] - PubMed
Royle 2003
-
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. - PubMed
Savovic 2012
-
- Savovic J, Jones HE, Altman DG, Harris RJ, Juni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [PUBMED: 22945832] - PubMed
Stata 13 [Computer program]
-
- Stata Corp. Stata 13. Texas: Stata Corp, 2007.
Stepanova 2012
-
- Stepanova M, Mishra A, Venkatesan C, Younossi ZM. In‐hospital mortality and economic burden associated with hepatic encephalopathy in the United States from 2005 to 2009. Clinical Gastroenterology and Hepatology 2012;10(9):1034‐41.e1. [PUBMED: 22642955] - PubMed
Thorlund 2011
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 23 April 2013).
TSA 2011 [Computer program]
-
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. Version 0.9 Beta. Copenhagen: Copenhagen Trial Unit, 2011.
Tygstrup 1984
-
- Tygstrup N, Vilstrup H. Effect of branched chain amino acids on the outcome of hepatic encephalopathy. Advances in Hepatic Encephalopathy and Urea Cycle Diseases. Basel: Karger, 1984:11‐4.
Wein 2004
-
- Wein C, Koch H, Popp B, Oehler G, Schauder P. Minimal hepatic encephalopathy impairs fitness to drive. Hepatology (Baltimore, Md.) 2004;39:739‐45. - PubMed
Weir 2012
-
- Weir MC, Grimshaw JM, Mayhew A, Fergusson D. Decisions about lumping vs. splitting of the scope of systematic reviews of complex interventions are not well justified: a case study in systematic reviews of health care professional reminders. Journal of Clinical Epidemiology 2012;65(7):756‐63. [PUBMED: 22498429] - PubMed
Weissenborn 2013
-
- Weissenborn K. Psychometric tests for diagnosing minimal hepatic encephalopathy. Metabolic Brain Disease 2013;28:227‐9. - PubMed
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [PUBMED: 18083463] - PubMed
Wetterslev 2009
Wood 2008
Wright 2007
-
- Wright G, Jalan R. Management of hepatic encephalopathy in patients with cirrhosis. Best Practice & Research Clinical Gastroenterology 2007;21:95‐110. - PubMed
References to other published versions of this review
Als‐Nielsen 2003
Gluud 2015a
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

