Access and response to direct antiviral agents (DAA) in HIV-HCV co-infected patients in Italy: Data from the Icona cohort
- PMID: 28520749
- PMCID: PMC5435319
- DOI: 10.1371/journal.pone.0177402
Access and response to direct antiviral agents (DAA) in HIV-HCV co-infected patients in Italy: Data from the Icona cohort
Abstract
Background: Real-life data on access and response to direct antiviral agents (DAA) in HIV-HCV coinfected individuals are lacking.
Methods: HCV viremic, HIV-positive patients from Icona and Hepaicona cohorts naïve to DAA by January 2013 were included. Access and predictors of starting DAA were evaluated. Switches of antiretroviral drugs at starting DAA were described. We calculated sustained virological response (SVR12) in those reaching 12 weeks after end-of-treatment (EOT), and defined treatment failure (TF) as discontinuation of DAA before EOT or non-SVR12. Statistical analyses included Kaplan-Meier curves, univariable and multivariable analyses evaluating predictors of access to DAA and of treatment outcome (non-SVR and TF).
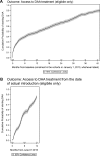
Results: 2,607 patients included. During a median follow-up of 38 (IQR:30-41) months, 920 (35.3%) patients started DAA. Eligibility for reimbursement was the strongest predictor to access to treatment: 761/1,090 (69.8%) eligible and 159/1,517 (10.5%) non-eligible to DAA reimbursement. Older age, HIV-RNA≤50 copies/mL were associated to faster DAA initiation, higher CD4 count and HCV-genotype 3 with delayed DAA initiation in those eligible to DAA reimbursement. Up to 28% of patients (36% of those on ritonavir-boosted protease inhibitors, PI/r) underwent antiretroviral (ART) modification at DAA initiation. 545/595 (91.6%) patients reaching EOT achieved SVR12. Overall, TF occurred in 61/606 patients (10.1%), with 11 discontinuing DAA before EOT. Suboptimal DAA was the only independent predictor of both non-SVR12 (AHR 2.52, 95%CI:1.24-5.12) and TF (AHR: 2.19; 95%CI:1.13-4.22).
Conclusions: Only 35.3% had access to HCV treatment. Despite excellent rates of SVR12 rates (91.6%), only 21% (545/2,607) of our HIV-HCV co-infected patients are cured.
Conflict of interest statement
Figures

Similar articles
-
Strategies for assignment of HIV-HCV genotype-1-coinfected patients to either dual-therapy or direct-acting antiviral agent-based triple-therapy.Antivir Ther. 2014;19(4):407-14. doi: 10.3851/IMP2717. Epub 2013 Dec 17. Antivir Ther. 2014. PMID: 24342953
-
Incidence and predictors of single drug discontinuation according to the presence of HCV coinfection in HIV patients from the ICONA Foundation Cohort Study.Eur J Clin Microbiol Infect Dis. 2018 May;37(5):871-881. doi: 10.1007/s10096-017-3180-8. Epub 2018 Jan 9. Eur J Clin Microbiol Infect Dis. 2018. PMID: 29318459
-
Sustained virological response after treatment with direct antiviral agents in individuals with HIV and hepatitis C co-infection.J Int AIDS Soc. 2022 Dec;25(12):e26048. doi: 10.1002/jia2.26048. J Int AIDS Soc. 2022. PMID: 36562643 Free PMC article.
-
Similar Sustained Virologic Response in Real-World and Clinical Trial Studies of Hepatitis C/Human Immunodeficiency Virus Coinfection.Dig Dis Sci. 2018 Nov;63(11):2829-2839. doi: 10.1007/s10620-018-5215-0. Epub 2018 Aug 9. Dig Dis Sci. 2018. PMID: 30094623 Review.
-
Direct-acting Antivirals for HIV/HCV Co-infected Individuals: As Good as it Gets?Curr HIV Res. 2017;15(6):422-433. doi: 10.2174/1570162X15666171108125255. Curr HIV Res. 2017. PMID: 29119934 Review.
Cited by
-
HIV screening and retention in care in people who use drugs in Madrid, Spain: a prospective study.Infect Dis Poverty. 2021 Aug 19;10(1):111. doi: 10.1186/s40249-021-00894-5. Infect Dis Poverty. 2021. PMID: 34412695 Free PMC article.
-
Predictors of Hepatitis C Treatment Failure After Using Direct-Acting Antivirals in People Living With Human Immunodeficiency Virus.Open Forum Infect Dis. 2019 Feb 11;6(3):ofz070. doi: 10.1093/ofid/ofz070. eCollection 2019 Mar. Open Forum Infect Dis. 2019. PMID: 30949524 Free PMC article.
-
Direct-acting Antiviral in the Treatment of Chronic Hepatitis C: Bonuses and Challenges.Int J Med Sci. 2020 Mar 15;17(7):892-902. doi: 10.7150/ijms.43079. eCollection 2020. Int J Med Sci. 2020. PMID: 32308542 Free PMC article. Review.
-
Estimation of the number of HCV-positive patients in Italy.PLoS One. 2019 Oct 31;14(10):e0223668. doi: 10.1371/journal.pone.0223668. eCollection 2019. PLoS One. 2019. PMID: 31671120 Free PMC article.
-
Liver Cirrhosis as a Risk Factor for Direct-Acting Antiviral Therapy Failure in Real-Life Hepatitis C Virus/Human Immunodeficiency Virus Coinfection.Open Forum Infect Dis. 2017 Jul 27;4(3):ofx158. doi: 10.1093/ofid/ofx158. eCollection 2017 Summer. Open Forum Infect Dis. 2017. PMID: 28948181 Free PMC article.
References
-
- McHutchison JG, Lawitz EJ, Shiffman ML, Muir AJ, Galler GW, McCone J, et al. Peginterferon alfa-2b or alfa-2° with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009;361:580–93. doi: 10.1056/NEJMoa0808010 - DOI - PubMed
-
- Hézode C, Fontaine H, Dorival C, Zoulim F, Larrey D, Canva V, et al. Effectiveness of telaprevir or boceprevir in treatment-experienced patients with HCV genotype 1 infection and cirrhosis. Gastroenterology. 2014;147(1):132–42 e4. doi: 10.1053/j.gastro.2014.03.051 - DOI - PubMed
-
- Sulkowski M, Pol S, Mallolas J, Fainboim H, Cooper C, Slim J, et al. Boceprevir versus placebo with pegylated interferon alfa-2b and ribavirin for treatment of hepatitis C virus genotype 1 in patients with HIV: a randomised, double-blind, controlled phase 2 trial. Lancet Infect Dis. 2013; 13(7):597–605. doi: 10.1016/S1473-3099(13)70149-X - DOI - PubMed
-
- Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for Previously Untreated Chronic Hepatitis C Infection. N Engl J Med. 2013; 368(20):1878–1887 doi: 10.1056/NEJMoa1214853 - DOI - PubMed
-
- Molina JM, Orkin C, Iser DM, Zamora FX, Nelson M, Stephan C, et al. Sofosbuvir plus ribavirin for treatment of hepatitis C virus in patients co-infected with HIV (PHOTON-2): a multicentre, open- label, non-randomised, phase 3 study. Lancet. 2015; 385(9973):1098–106. doi: 10.1016/S0140-6736(14)62483-1 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

