Texture analysis using proton density and T2 relaxation in patients with histological usual interstitial pneumonia (UIP) or nonspecific interstitial pneumonia (NSIP)
- PMID: 28520778
- PMCID: PMC5433738
- DOI: 10.1371/journal.pone.0177689
Texture analysis using proton density and T2 relaxation in patients with histological usual interstitial pneumonia (UIP) or nonspecific interstitial pneumonia (NSIP)
Abstract
Objectives: The purpose of our study was to assess proton density (PD) and T2 relaxation time of usual interstitial pneumonia (UIP) and nonspecific interstitial pneumonia (NSIP) and to evaluate their utility in differentiating the two patterns. Furthermore, we aim to investigate whether these two parameters could help differentiate active-inflammatory and stable-fibrotic lesions in NSIP.
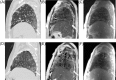
Methods: 32 patients (mean age: 69 years; M:F, 1:1) with pathologically proven disease (UIP:NSIP, 1:1), underwent thoracic thin-section multislice CT scan and 1.5T MRI. A total of 437 regions-of-interest (ROIs) were classified at CT as advanced, moderate or mild alterations. Based on multi-echo single-shot TSE sequence acquired at five echo times, with breath-holding at end-expiration and ECG-triggering, entire lung T2 and PD maps were generated from each subject. The T2 relaxation time and the respective signal intensity were quantified by performing a ROI measurement on the T2 and PD maps in the corresponding CT selected areas of the lung.
Results: UIP and NSIP regional patterns could not be differentiated by T2 relaxation times or PD values alone. Overall, a strong positive correlation was found between T2 relaxation and PD in NSIP, r = 0.64, p<0.001; however, this correlation was weak in UIP, r = 0.20, p = 0.01. T2 relaxation showed significant statistical difference between active-inflammatory and stable-fibrotic NSIP regions at all levels, p<0.05, while for the analysis of ventral lesions PD proved no statistical difference, p>0.05.
Conclusions: T2 relaxation times and PD values may provide helpful quantitative information for differentiating NSIP from UIP pattern. These parameters have the potential to differentiate active-inflammatory and stable-fibrotic lesions in NSIP.
Conflict of interest statement
Figures
References
-
- Larsen BT, Colby TV. Update for pathologists on idiopathic interstitial pneumonias. Arch Pathol Lab Med. 2012;136(10):1234–41. doi: 10.5858/arpa.2012-0225-RA - DOI - PubMed
-
- Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis—evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL - DOI - PMC - PubMed
-
- Travis WD, Hunninghake G, King TE Jr, Lynch DA, Colby TV, Galvin JR, et al. Idiopathic nonspecific interstitial pneumonia: report of an American Thoracic Society project. Am J Respir Crit Care Med. 2008;177(12):1338–47. doi: 10.1164/rccm.200611-1685OC - DOI - PubMed
-
- Katzenstein AL, Fiorelli RF. Nonspecific interstitial pneumonia/fibrosis. Histologic features and clinical significance. Am J Surg Pathol. 1994;18(2):136–47. - PubMed
-
- Hodnett PA, Naidich DP. Fibrosing interstitial lung disease: A practical HRCT based approach to diagnosis and management and review of the literature. Am J Respir Crit Care Med. 2013;188(2):141–9. doi: 10.1164/rccm.201208-1544CI - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

